| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1163037 | 1490926 | 2016 | 8 صفحه PDF | دانلود رایگان |
• A sensitive and versatile ECL biosensing platform is developed for monitoring protein kinase activity and inhibition.
• Ru(bpy)32+ functionalized gold nanoparticles are used as thiol-versatile signal probe.
• The strategy exhibits unique advantages of high sensitivity, good selectivity and versatility.
• The strategy is promising for multiple protein kinase assay and kinase inhibitor profiling.
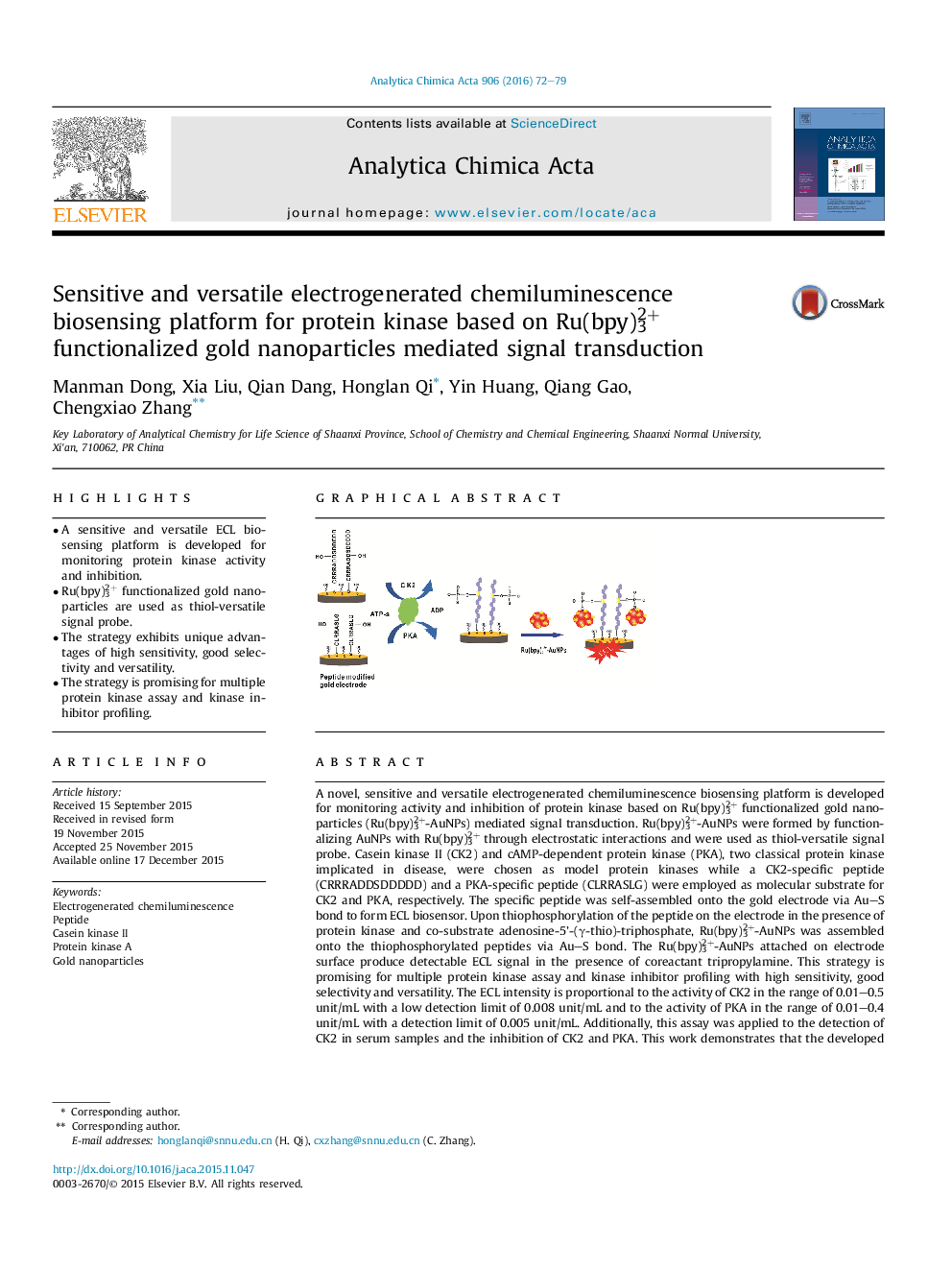
A novel, sensitive and versatile electrogenerated chemiluminescence biosensing platform is developed for monitoring activity and inhibition of protein kinase based on Ru(bpy)32+ functionalized gold nanoparticles (Ru(bpy)32+-AuNPs) mediated signal transduction. Ru(bpy)32+-AuNPs were formed by functionalizing AuNPs with Ru(bpy)32+ through electrostatic interactions and were used as thiol-versatile signal probe. Casein kinase II (CK2) and cAMP-dependent protein kinase (PKA), two classical protein kinase implicated in disease, were chosen as model protein kinases while a CK2-specific peptide (CRRRADDSDDDDD) and a PKA-specific peptide (CLRRASLG) were employed as molecular substrate for CK2 and PKA, respectively. The specific peptide was self-assembled onto the gold electrode via Au–S bond to form ECL biosensor. Upon thiophosphorylation of the peptide on the electrode in the presence of protein kinase and co-substrate adenosine-5’-(γ-thio)-triphosphate, Ru(bpy)32+-AuNPs was assembled onto the thiophosphorylated peptides via Au–S bond. The Ru(bpy)32+-AuNPs attached on electrode surface produce detectable ECL signal in the presence of coreactant tripropylamine. This strategy is promising for multiple protein kinase assay and kinase inhibitor profiling with high sensitivity, good selectivity and versatility. The ECL intensity is proportional to the activity of CK2 in the range of 0.01–0.5 unit/mL with a low detection limit of 0.008 unit/mL and to the activity of PKA in the range of 0.01–0.4 unit/mL with a detection limit of 0.005 unit/mL. Additionally, this assay was applied to the detection of CK2 in serum samples and the inhibition of CK2 and PKA. This work demonstrates that the developed ECL method can provide a sensitive and versatile platform for the detection of kinase activity and drug-screening.
Figure optionsDownload as PowerPoint slide
Journal: Analytica Chimica Acta - Volume 906, 4 February 2016, Pages 72–79