| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1232485 | 968792 | 2015 | 8 صفحه PDF | دانلود رایگان |
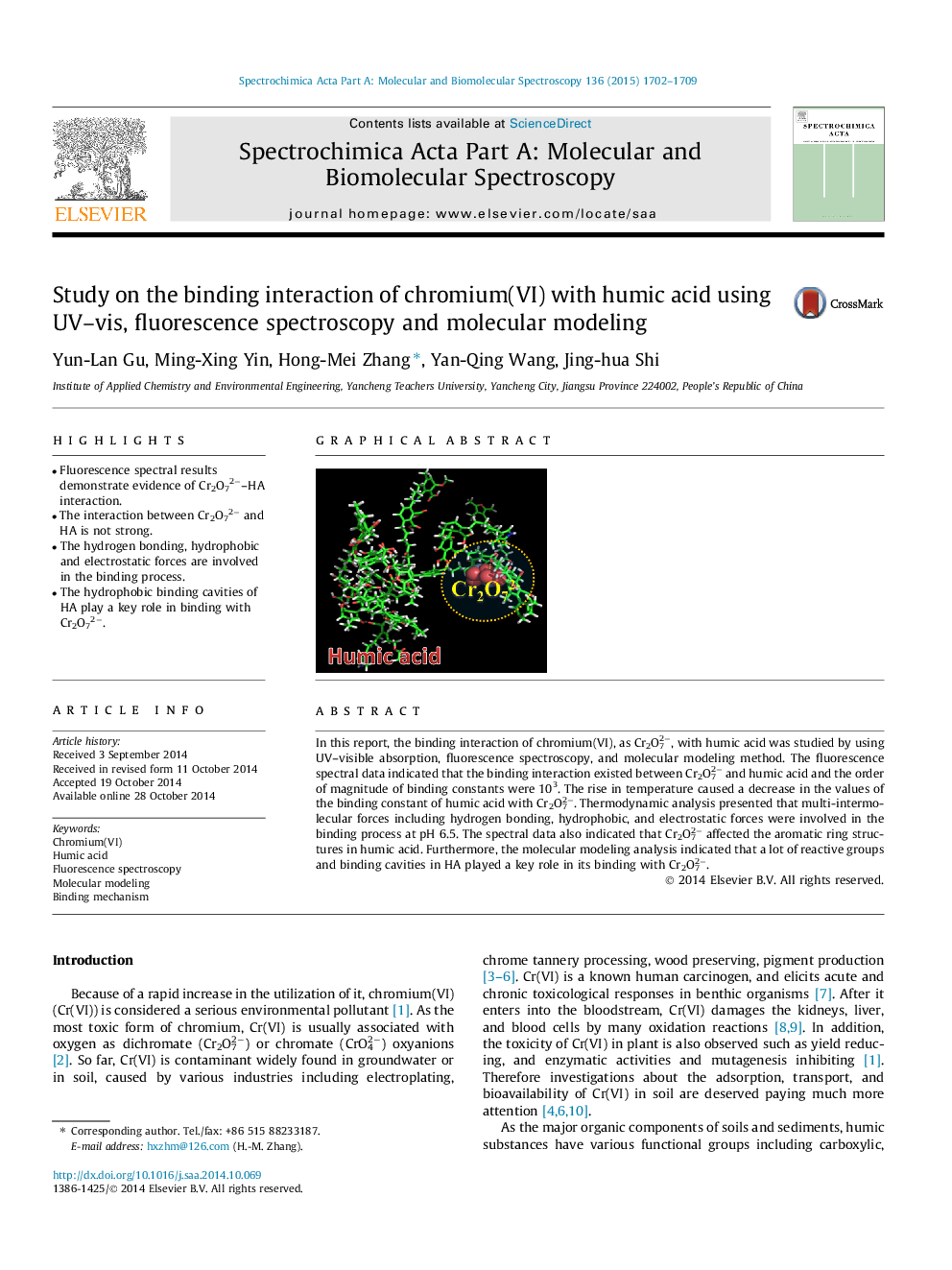
• Fluorescence spectral results demonstrate evidence of Cr2O72−–HA interaction.
• The interaction between Cr2O72− and HA is not strong.
• The hydrogen bonding, hydrophobic and electrostatic forces are involved in the binding process.
• The hydrophobic binding cavities of HA play a key role in binding with Cr2O72−.
In this report, the binding interaction of chromium(VI), as Cr2O72−, with humic acid was studied by using UV–visible absorption, fluorescence spectroscopy, and molecular modeling method. The fluorescence spectral data indicated that the binding interaction existed between Cr2O72− and humic acid and the order of magnitude of binding constants were 103. The rise in temperature caused a decrease in the values of the binding constant of humic acid with Cr2O72−. Thermodynamic analysis presented that multi-intermolecular forces including hydrogen bonding, hydrophobic, and electrostatic forces were involved in the binding process at pH 6.5. The spectral data also indicated that Cr2O72− affected the aromatic ring structures in humic acid. Furthermore, the molecular modeling analysis indicated that a lot of reactive groups and binding cavities in HA played a key role in its binding with Cr2O72−.
Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 136, Part C, 5 February 2015, Pages 1702–1709