| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1390234 | 1500870 | 2013 | 9 صفحه PDF | دانلود رایگان |
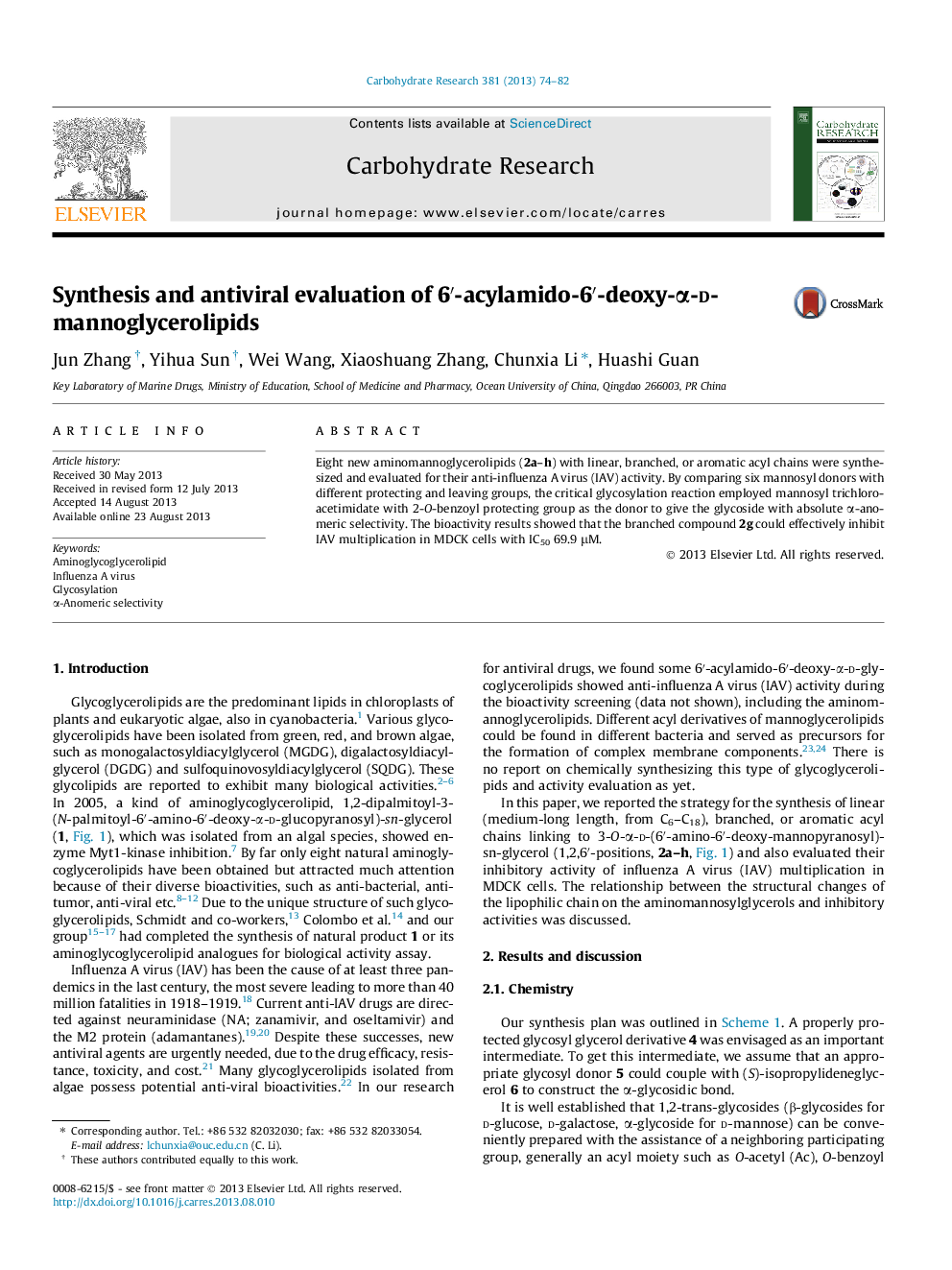
• The 6′-acylamido-6′-deoxy-α-d-mannoglycerolipids 2a–h were synthesized with a concise strategy.
• Glycosylation employed donor 5d with 2-O-benzoyl group achieved absolute α-selectivity.
• The compound 2d inhibited IAV multiplication in MDCK cells effectively.
Eight new aminomannoglycerolipids (2a–h) with linear, branched, or aromatic acyl chains were synthesized and evaluated for their anti-influenza A virus (IAV) activity. By comparing six mannosyl donors with different protecting and leaving groups, the critical glycosylation reaction employed mannosyl trichloroacetimidate with 2-O-benzoyl protecting group as the donor to give the glycoside with absolute α-anomeric selectivity. The bioactivity results showed that the branched compound 2g could effectively inhibit IAV multiplication in MDCK cells with IC50 69.9 μM.
Figure optionsDownload as PowerPoint slide
Journal: Carbohydrate Research - Volume 381, 15 November 2013, Pages 74–82