| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1402657 | 1501753 | 2014 | 8 صفحه PDF | دانلود رایگان |
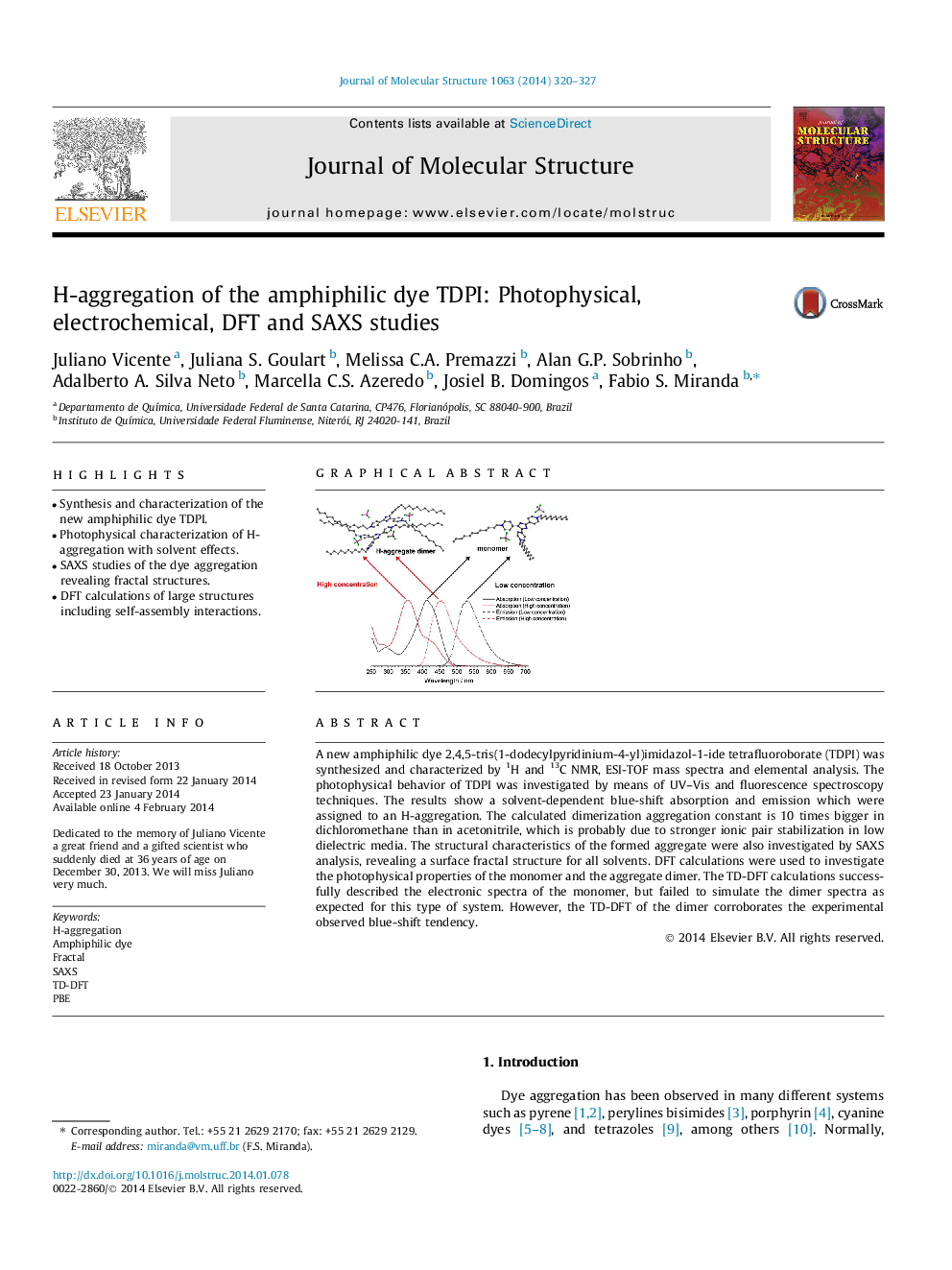
• Synthesis and characterization of the new amphiphilic dye TDPI.
• Photophysical characterization of H-aggregation with solvent effects.
• SAXS studies of the dye aggregation revealing fractal structures.
• DFT calculations of large structures including self-assembly interactions.
A new amphiphilic dye 2,4,5-tris(1-dodecylpyridinium-4-yl)imidazol-1-ide tetrafluoroborate (TDPI) was synthesized and characterized by 1H and 13C NMR, ESI-TOF mass spectra and elemental analysis. The photophysical behavior of TDPI was investigated by means of UV–Vis and fluorescence spectroscopy techniques. The results show a solvent-dependent blue-shift absorption and emission which were assigned to an H-aggregation. The calculated dimerization aggregation constant is 10 times bigger in dichloromethane than in acetonitrile, which is probably due to stronger ionic pair stabilization in low dielectric media. The structural characteristics of the formed aggregate were also investigated by SAXS analysis, revealing a surface fractal structure for all solvents. DFT calculations were used to investigate the photophysical properties of the monomer and the aggregate dimer. The TD-DFT calculations successfully described the electronic spectra of the monomer, but failed to simulate the dimer spectra as expected for this type of system. However, the TD-DFT of the dimer corroborates the experimental observed blue-shift tendency.
Figure optionsDownload as PowerPoint slide
Journal: Journal of Molecular Structure - Volume 1063, 24 April 2014, Pages 320–327