| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 145857 | 456352 | 2016 | 7 صفحه PDF | دانلود رایگان |
• Three functionalized MOFs were designed under the guidance of the QSPR model.
• CO2 capture ability is greatly enhanced by introducing –2SO3H group into the MOF.
• Experimental results show the QSPR model is a useful tool for the MOF design.
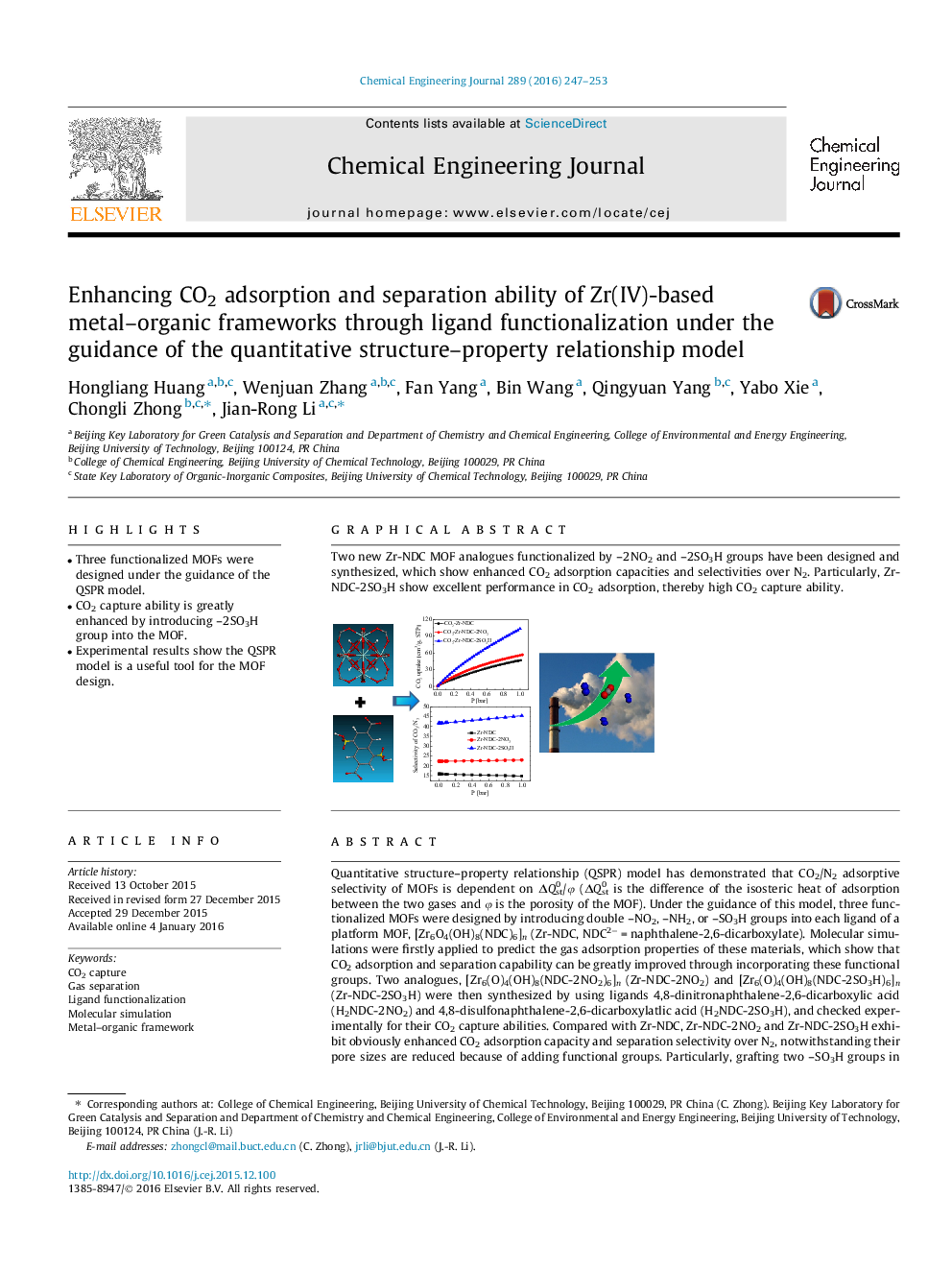
Quantitative structure–property relationship (QSPR) model has demonstrated that CO2/N2 adsorptive selectivity of MOFs is dependent on ΔQst0/φ (ΔQst0 is the difference of the isosteric heat of adsorption between the two gases and φ is the porosity of the MOF). Under the guidance of this model, three functionalized MOFs were designed by introducing double –NO2, –NH2, or –SO3H groups into each ligand of a platform MOF, [Zr6O4(OH)8(NDC)6]n (Zr-NDC, NDC2− = naphthalene-2,6-dicarboxylate). Molecular simulations were firstly applied to predict the gas adsorption properties of these materials, which show that CO2 adsorption and separation capability can be greatly improved through incorporating these functional groups. Two analogues, [Zr6(O)4(OH)8(NDC-2NO2)6]n (Zr-NDC-2NO2) and [Zr6(O)4(OH)8(NDC-2SO3H)6]n (Zr-NDC-2SO3H) were then synthesized by using ligands 4,8-dinitronaphthalene-2,6-dicarboxylic acid (H2NDC-2NO2) and 4,8-disulfonaphthalene-2,6-dicarboxylatlic acid (H2NDC-2SO3H), and checked experimentally for their CO2 capture abilities. Compared with Zr-NDC, Zr-NDC-2NO2 and Zr-NDC-2SO3H exhibit obviously enhanced CO2 adsorption capacity and separation selectivity over N2, notwithstanding their pore sizes are reduced because of adding functional groups. Particularly, grafting two –SO3H groups in each ligand has led to a double gravimetric and triple volumetric increase of CO2 adsorption capacity and a very high CO2/N2 separation selectivity in Zr-NDC-2SO3H. Usually, the more functional groups in pores of a MOF are, the better their efficiency is. In these functionalized MOFs, the density of the functional groups is quite high, thus a huge impact on the gas adsorption property was achieved. These results also suggest that the QSPR model is a useful tool in designing MOFs with high performances for CO2 capture.
Two new Zr-NDC MOF analogues functionalized by –2NO2 and –2SO3H groups have been designed and synthesized, which show enhanced CO2 adsorption capacities and selectivities over N2. Particularly, Zr-NDC-2SO3H show excellent performance in CO2 adsorption, thereby high CO2 capture ability.Figure optionsDownload as PowerPoint slide
Journal: Chemical Engineering Journal - Volume 289, 1 April 2016, Pages 247–253