| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5370710 | 1503902 | 2016 | 6 صفحه PDF | دانلود رایگان |
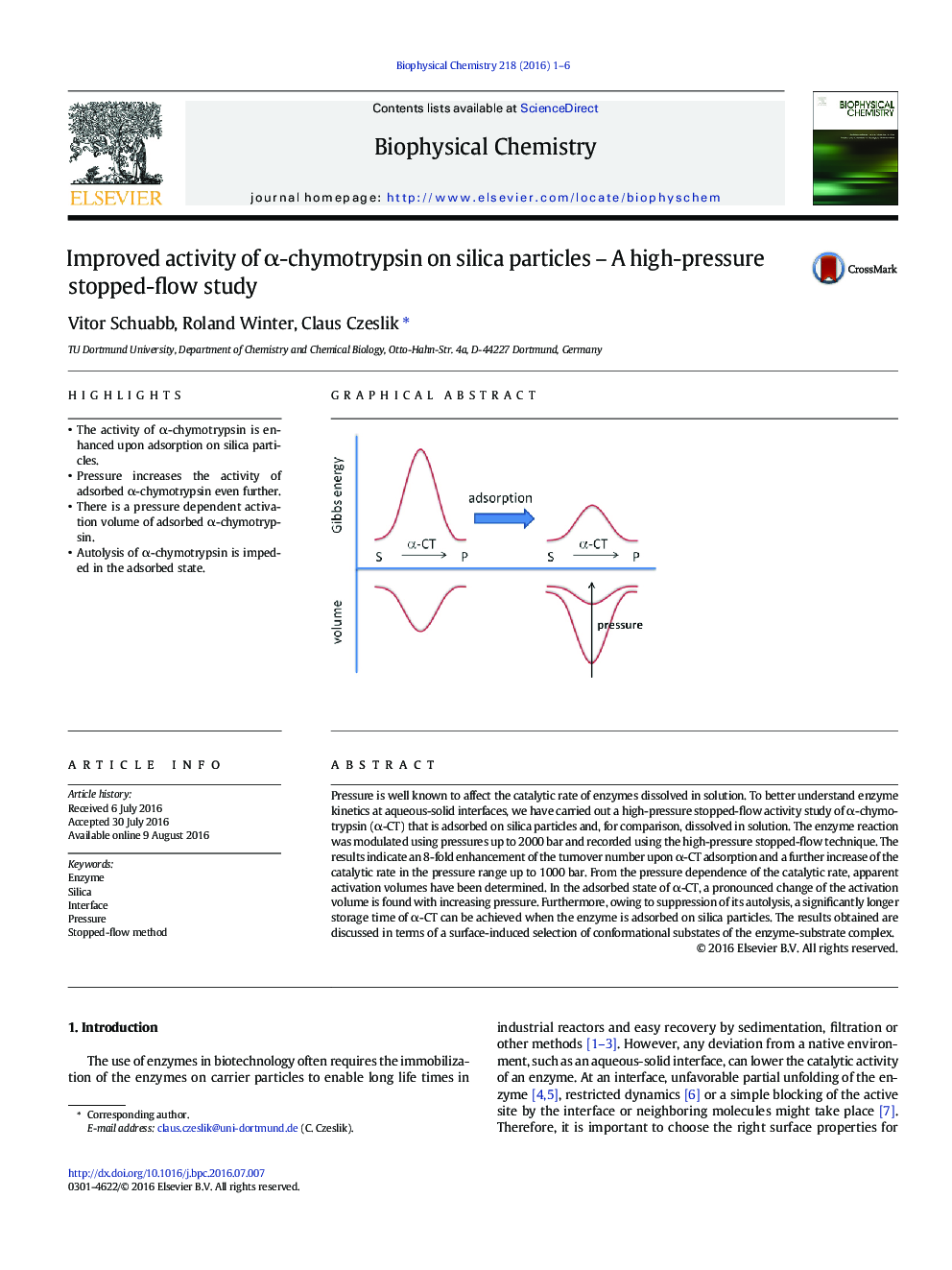
- The activity of α-chymotrypsin is enhanced upon adsorption on silica particles.
- Pressure increases the activity of adsorbed α-chymotrypsin even further.
- There is a pressure dependent activation volume of adsorbed α-chymotrypsin.
- Autolysis of α-chymotrypsin is impeded in the adsorbed state.
Pressure is well known to affect the catalytic rate of enzymes dissolved in solution. To better understand enzyme kinetics at aqueous-solid interfaces, we have carried out a high-pressure stopped-flow activity study of α-chymotrypsin (α-CT) that is adsorbed on silica particles and, for comparison, dissolved in solution. The enzyme reaction was modulated using pressures up to 2000 bar and recorded using the high-pressure stopped-flow technique. The results indicate an 8-fold enhancement of the turnover number upon α-CT adsorption and a further increase of the catalytic rate in the pressure range up to 1000 bar. From the pressure dependence of the catalytic rate, apparent activation volumes have been determined. In the adsorbed state of α-CT, a pronounced change of the activation volume is found with increasing pressure. Furthermore, owing to suppression of its autolysis, a significantly longer storage time of α-CT can be achieved when the enzyme is adsorbed on silica particles. The results obtained are discussed in terms of a surface-induced selection of conformational substates of the enzyme-substrate complex.
51
Journal: Biophysical Chemistry - Volume 218, November 2016, Pages 1-6