| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 53779 | 46984 | 2016 | 7 صفحه PDF | دانلود رایگان |
• MnO2 catalysts were prepared using a simple redox method at room temperature.
• Primary alcohol rapidly produced high surface area MnO2via the reduction of MnO4−.
• MnO2 prepared with 1-butanol contains the most abundant lattice oxygen species.
• The physicochemical properties of alcohols can control properties of prepared MnO2.
MnO2 catalysts were synthesized in an aqueous solution of KMnO4 and C2–C5 alcohols using a simple redox method at room temperature. The crystalline structure of all samples was δ-MnO2 after being calcined at 300 °C. However, other physicochemical properties of the samples varied depending on the symmetry of the alcohols used. For the catalytic oxidation of CO, MnO2 catalysts prepared with 1° alcohols performed better than the samples prepared in 2° alcohols. Catalytic activities were correlated to the quantity of labile oxygen species of the catalysts. In CO-TPD analysis, the relative area of desorbed CO2, which is the product of the reaction between adsorbed CO and lattice oxygen species, becomes larger for MnO2 prepared with 1° alcohols than with 2° alcohols. These results were primarily resulted from the innate hydrogen dissociation behavior of alcohol in solution. The pKa was found to be an important factor in determining the physicochemical properties and catalytic activity toward CO oxidation of MnO2.
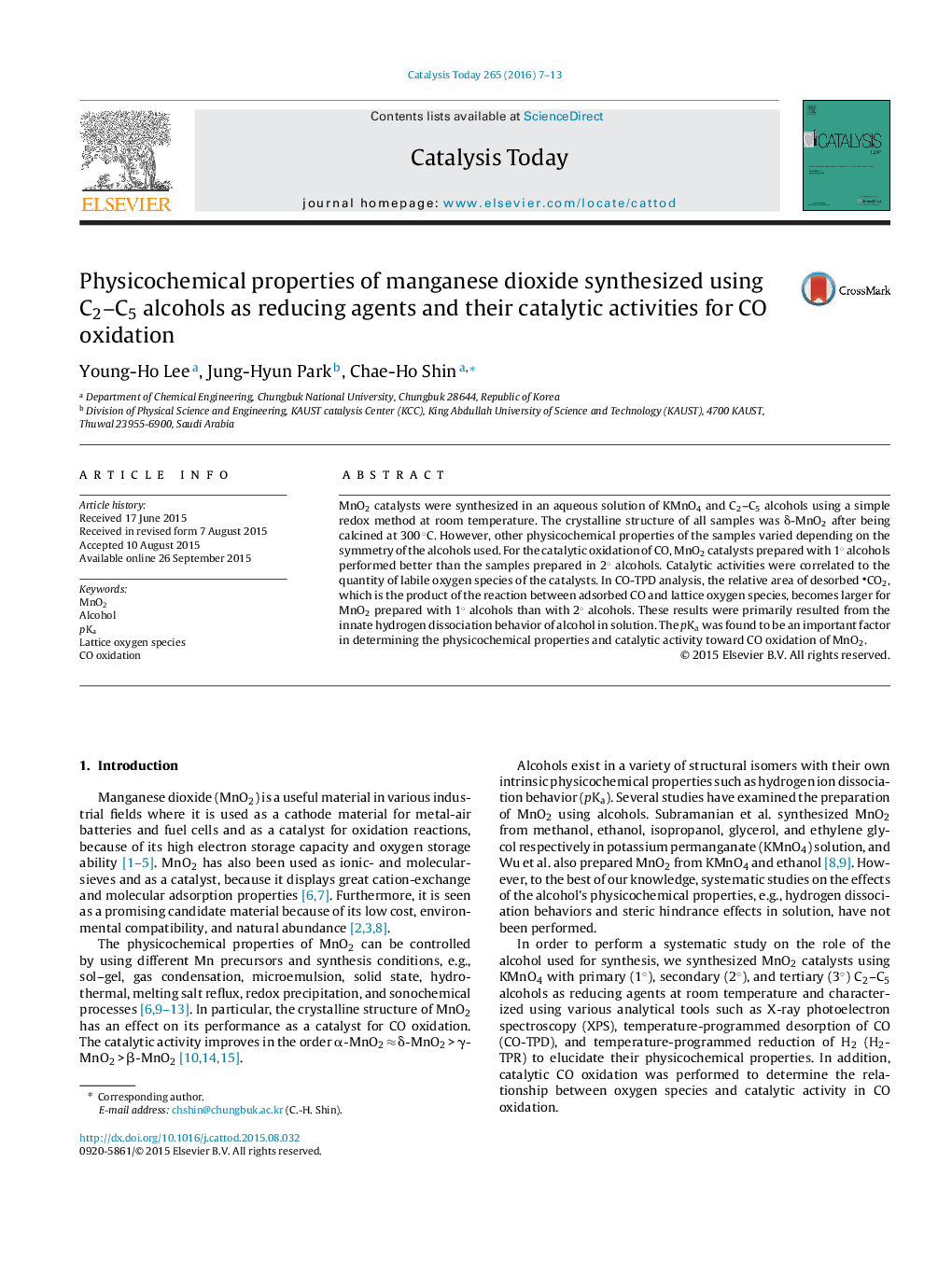
Correlation between the catalytic CO oxidation activity and quantity of lattice oxygen species analyzed by CO-TPD and XPS. Tlight-off is the temperature at which 50% CO conversion is achieved.Figure optionsDownload high-quality image (137 K)Download as PowerPoint slide
Journal: Catalysis Today - Volume 265, 1 May 2016, Pages 7–13