| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 61023 | 47561 | 2014 | 6 صفحه PDF | دانلود رایگان |
• Au catalysts are shown to be active for photocatalytic reforming of a variety of alcohols.
• Au is less active than Pd.
• Au–Pd alloys appear to be a little more active than either individual component.
• There is a maximum in activity at low loadings, and high loadings lead to much diminished activity.
• Incipient wetness impregnation appears to be a good method for the preparation of such catalysts.
We have prepared a variety of Au, Pd and Au–Pd catalysts loaded onto P25 titania to determine how effective these materials are for the anaerobic, ambient temperature reforming of alcohols with water to produce hydrogen, and to examine the effect of the preparation method on their performance. Catalysts produced by both incipient wetness (IW) methods and by colloidal methods are successful materials for producing hydrogen, with the IW catalysts proving to be the best for the same metal loading. It is shown that, although gold catalysts generally have lower hydrogen yield than for Pd, alloying Au with Pd gives more active materials than either alone at the same weight loading, due to a synergistic effect. The catalysts are active for reforming a range of alcohols, generally producing CO2, H2 and an alkane. However, it is essential for good activity to have an H at the α-position to the oxygenate function, and so, carboxylic acids and ketones do not work. These catalysts generally show a maximum in activity at low loadings of metal (∼0.5 wt%) due to a requirement for maximising the active interface between the metal nanoparticles and the photo-active titania.
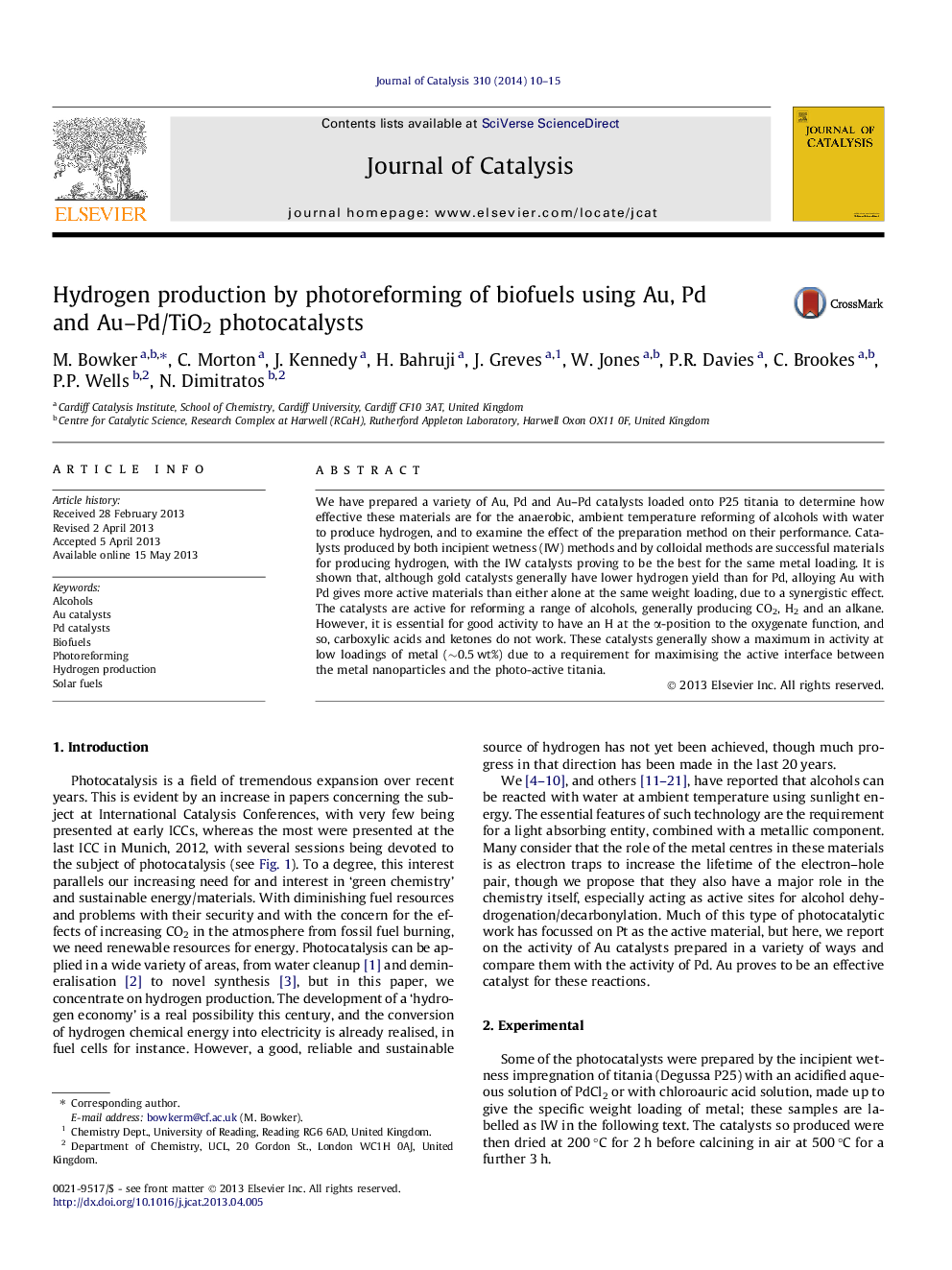
The photocatalytic reforming of methanol. Step 1, adsorption of methanol; step 2, dissociation of methanol and hydrogen evolution; step 3, photo-excitation of an electron–hole pair, thus creating a highly reactive, electrophilic oxygen species, which then, step 4 oxidises adsorbed CO to gas phase CO2. Not shown here is the subsequent filling of the remaining anion vacancy in the titania lattice by reaction with water.Figure optionsDownload high-quality image (58 K)Download as PowerPoint slide
Journal: Journal of Catalysis - Volume 310, February 2014, Pages 10–15