| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 6455254 | 1419172 | 2017 | 9 صفحه PDF | دانلود رایگان |
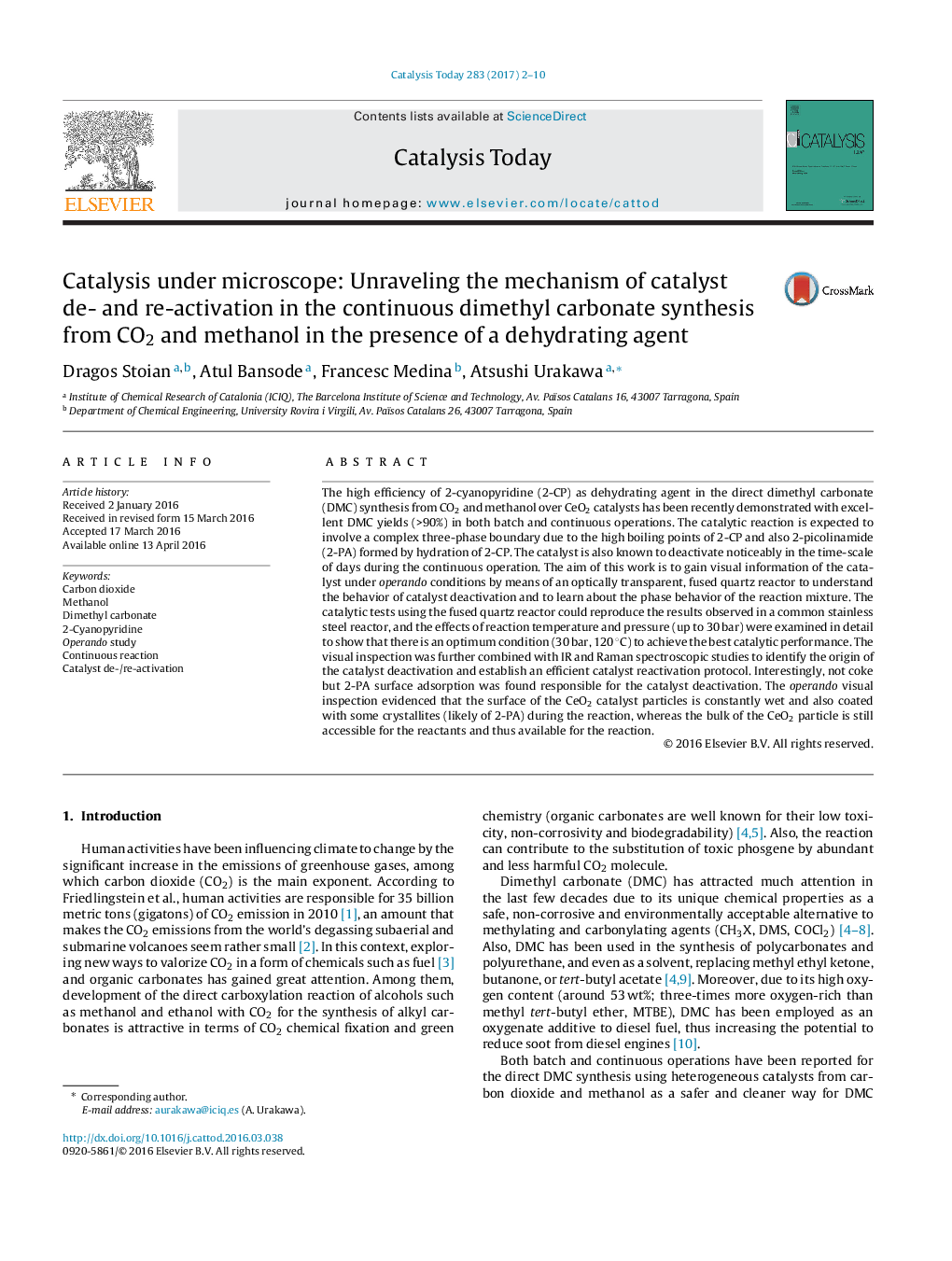
- Visually looking into CeO2 catalyst in DMC synthesis from CO2 & methanol (â¤30 bar).
- Using an organic dehydrating agent, thus a complex three-phase boundary was formed.
- Rapid color change of the catalyst with slower deactivation.
- Strong binding of 2-picolinamide over CeO2 causes the catalyst deactivation.
- Mild temperature (300 °C) calcination can reactivate the catalyst completely.
The high efficiency of 2-cyanopyridine (2-CP) as dehydrating agent in the direct dimethyl carbonate (DMC) synthesis from CO2 and methanol over CeO2 catalysts has been recently demonstrated with excellent DMC yields (>90%) in both batch and continuous operations. The catalytic reaction is expected to involve a complex three-phase boundary due to the high boiling points of 2-CP and also 2-picolinamide (2-PA) formed by hydration of 2-CP. The catalyst is also known to deactivate noticeably in the time-scale of days during the continuous operation. The aim of this work is to gain visual information of the catalyst under operando conditions by means of an optically transparent, fused quartz reactor to understand the behavior of catalyst deactivation and to learn about the phase behavior of the reaction mixture. The catalytic tests using the fused quartz reactor could reproduce the results observed in a common stainless steel reactor, and the effects of reaction temperature and pressure (up to 30 bar) were examined in detail to show that there is an optimum condition (30 bar, 120 °C) to achieve the best catalytic performance. The visual inspection was further combined with IR and Raman spectroscopic studies to identify the origin of the catalyst deactivation and establish an efficient catalyst reactivation protocol. Interestingly, not coke but 2-PA surface adsorption was found responsible for the catalyst deactivation. The operando visual inspection evidenced that the surface of the CeO2 catalyst particles is constantly wet and also coated with some crystallites (likely of 2-PA) during the reaction, whereas the bulk of the CeO2 particle is still accessible for the reactants and thus available for the reaction.
129
Journal: Catalysis Today - Volume 283, 1 April 2017, Pages 2-10