| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 6465239 | 1422950 | 2017 | 8 صفحه PDF | دانلود رایگان |
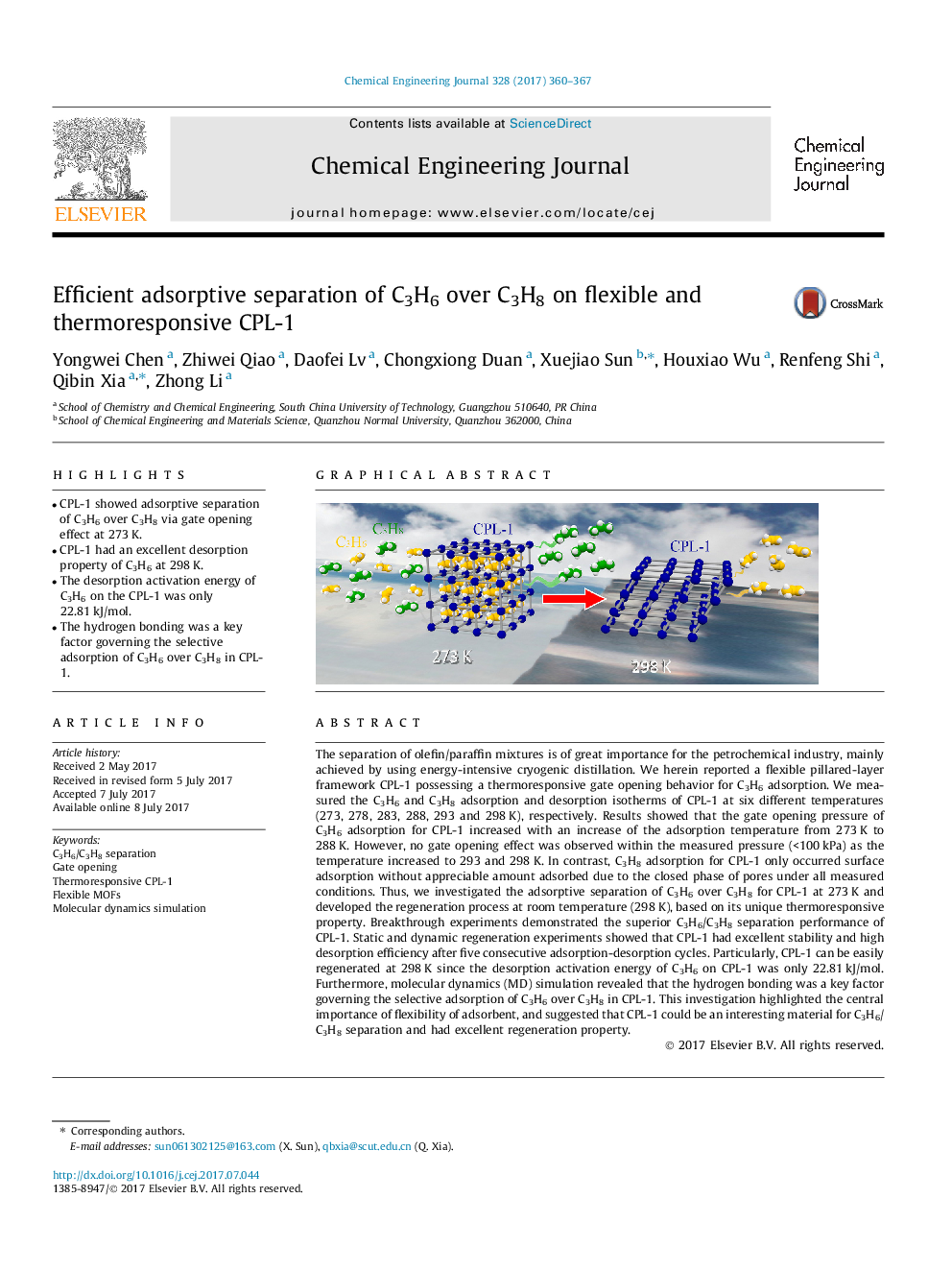
- CPL-1 showed adsorptive separation of C3H6 over C3H8 via gate opening effect at 273Â K.
- CPL-1 had an excellent desorption property of C3H6 at 298Â K.
- The desorption activation energy of C3H6 on the CPL-1 was only 22.81Â kJ/mol.
- The hydrogen bonding was a key factor governing the selective adsorption of C3H6 over C3H8 in CPL-1.
The separation of olefin/paraffin mixtures is of great importance for the petrochemical industry, mainly achieved by using energy-intensive cryogenic distillation. We herein reported a flexible pillared-layer framework CPL-1 possessing a thermoresponsive gate opening behavior for C3H6 adsorption. We measured the C3H6 and C3H8 adsorption and desorption isotherms of CPL-1 at six different temperatures (273, 278, 283, 288, 293 and 298Â K), respectively. Results showed that the gate opening pressure of C3H6 adsorption for CPL-1 increased with an increase of the adsorption temperature from 273Â K to 288Â K. However, no gate opening effect was observed within the measured pressure (<100Â kPa) as the temperature increased to 293 and 298Â K. In contrast, C3H8 adsorption for CPL-1 only occurred surface adsorption without appreciable amount adsorbed due to the closed phase of pores under all measured conditions. Thus, we investigated the adsorptive separation of C3H6 over C3H8 for CPL-1 at 273Â K and developed the regeneration process at room temperature (298Â K), based on its unique thermoresponsive property. Breakthrough experiments demonstrated the superior C3H6/C3H8 separation performance of CPL-1. Static and dynamic regeneration experiments showed that CPL-1 had excellent stability and high desorption efficiency after five consecutive adsorption-desorption cycles. Particularly, CPL-1 can be easily regenerated at 298Â K since the desorption activation energy of C3H6 on CPL-1 was only 22.81Â kJ/mol. Furthermore, molecular dynamics (MD) simulation revealed that the hydrogen bonding was a key factor governing the selective adsorption of C3H6 over C3H8 in CPL-1. This investigation highlighted the central importance of flexibility of adsorbent, and suggested that CPL-1 could be an interesting material for C3H6/C3H8 separation and had excellent regeneration property.
231
Journal: Chemical Engineering Journal - Volume 328, 15 November 2017, Pages 360-367