| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 6465522 | 1422952 | 2017 | 15 صفحه PDF | دانلود رایگان |
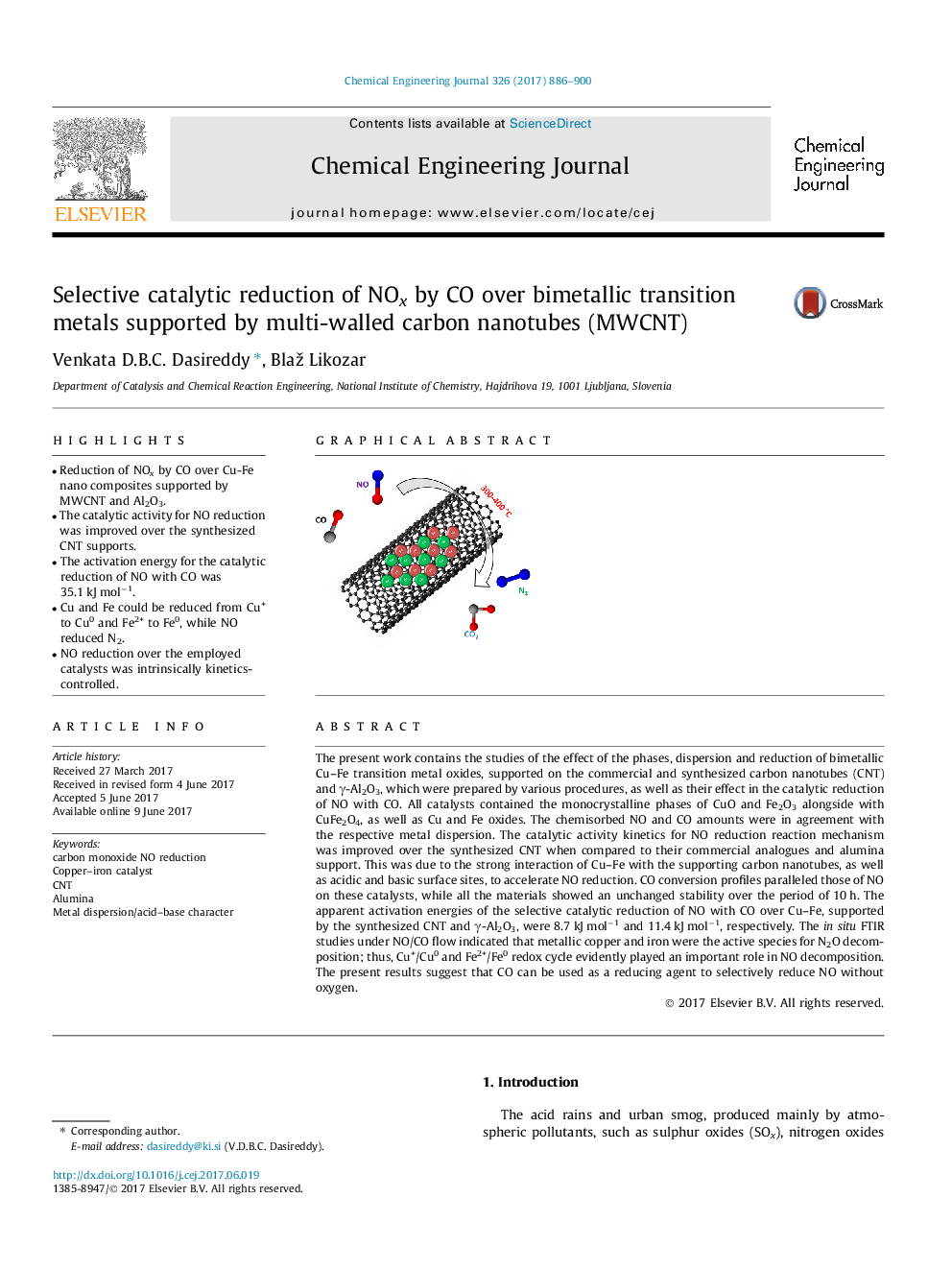
- Reduction of NOx by CO over Cu-Fe nano composites supported by MWCNT and Al2O3.
- The catalytic activity for NO reduction was improved over the synthesized CNT supports.
- The activation energy for the catalytic reduction of NO with CO was 35.1Â kJÂ molâ1.
- Cu and Fe could be reduced from Cu+ to Cu0 and Fe2+ to Fe0, while NO reduced N2.
- NO reduction over the employed catalysts was intrinsically kinetics-controlled.
The present work contains the studies of the effect of the phases, dispersion and reduction of bimetallic Cu-Fe transition metal oxides, supported on the commercial and synthesized carbon nanotubes (CNT) and γ-Al2O3, which were prepared by various procedures, as well as their effect in the catalytic reduction of NO with CO. All catalysts contained the monocrystalline phases of CuO and Fe2O3 alongside with CuFe2O4, as well as Cu and Fe oxides. The chemisorbed NO and CO amounts were in agreement with the respective metal dispersion. The catalytic activity kinetics for NO reduction reaction mechanism was improved over the synthesized CNT when compared to their commercial analogues and alumina support. This was due to the strong interaction of Cu-Fe with the supporting carbon nanotubes, as well as acidic and basic surface sites, to accelerate NO reduction. CO conversion profiles paralleled those of NO on these catalysts, while all the materials showed an unchanged stability over the period of 10 h. The apparent activation energies of the selective catalytic reduction of NO with CO over Cu-Fe, supported by the synthesized CNT and γ-Al2O3, were 8.7 kJ molâ1 and 11.4 kJ molâ1, respectively. The in situ FTIR studies under NO/CO flow indicated that metallic copper and iron were the active species for N2O decomposition; thus, Cu+/Cu0 and Fe2+/Fe0 redox cycle evidently played an important role in NO decomposition. The present results suggest that CO can be used as a reducing agent to selectively reduce NO without oxygen.
62
Journal: Chemical Engineering Journal - Volume 326, 15 October 2017, Pages 886-900