| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 6466183 | 1422953 | 2017 | 13 صفحه PDF | دانلود رایگان |
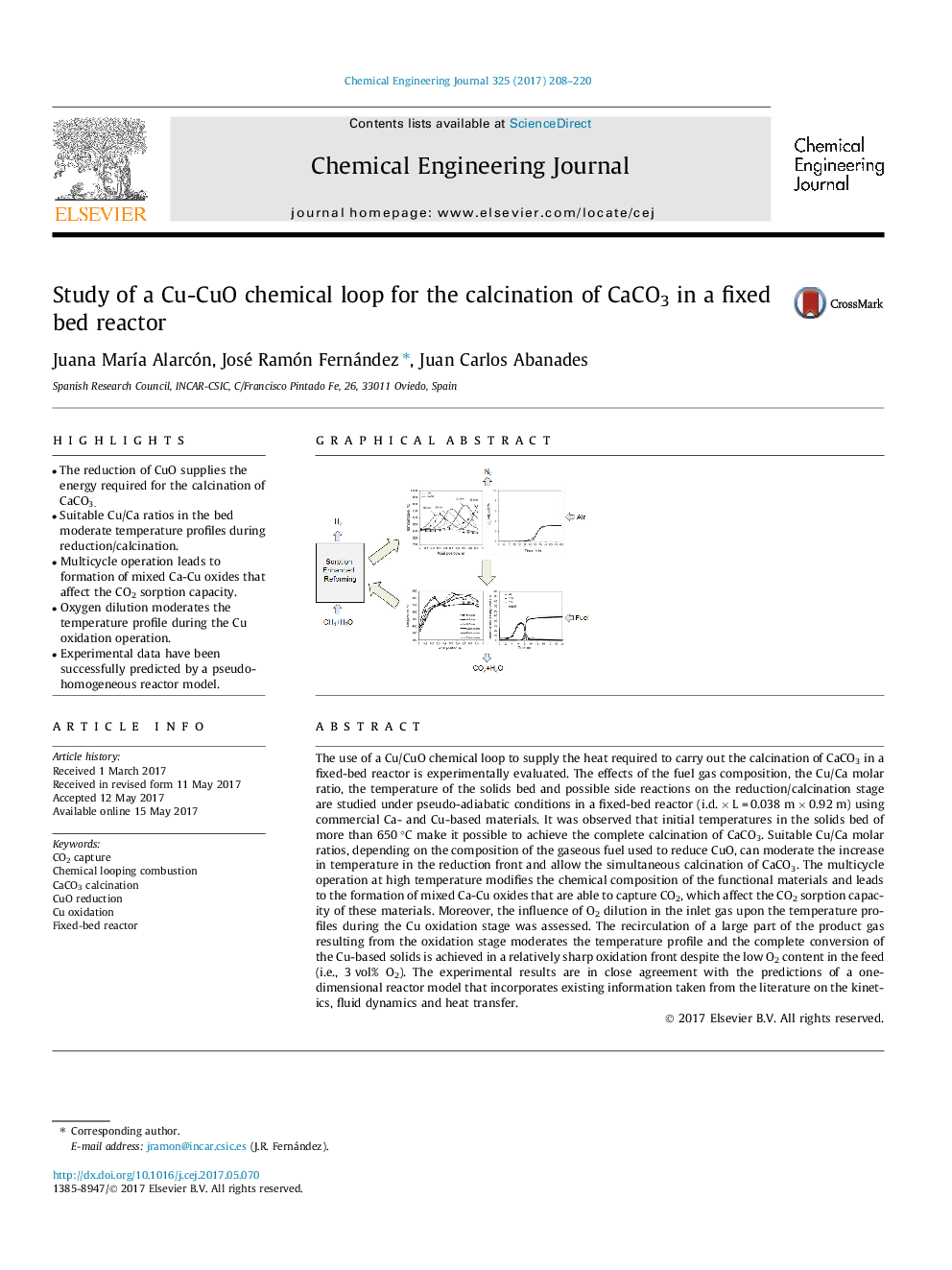
- The reduction of CuO supplies the energy required for the calcination of CaCO3.
- Suitable Cu/Ca ratios in the bed moderate temperature profiles during reduction/calcination.
- Multicycle operation leads to formation of mixed Ca-Cu oxides that affect the CO2 sorption capacity.
- Oxygen dilution moderates the temperature profile during the Cu oxidation operation.
- Experimental data have been successfully predicted by a pseudo-homogeneous reactor model.
The use of a Cu/CuO chemical loop to supply the heat required to carry out the calcination of CaCO3 in a fixed-bed reactor is experimentally evaluated. The effects of the fuel gas composition, the Cu/Ca molar ratio, the temperature of the solids bed and possible side reactions on the reduction/calcination stage are studied under pseudo-adiabatic conditions in a fixed-bed reactor (i.d. Ã L = 0.038 m Ã 0.92 m) using commercial Ca- and Cu-based materials. It was observed that initial temperatures in the solids bed of more than 650 °C make it possible to achieve the complete calcination of CaCO3. Suitable Cu/Ca molar ratios, depending on the composition of the gaseous fuel used to reduce CuO, can moderate the increase in temperature in the reduction front and allow the simultaneous calcination of CaCO3. The multicycle operation at high temperature modifies the chemical composition of the functional materials and leads to the formation of mixed Ca-Cu oxides that are able to capture CO2, which affect the CO2 sorption capacity of these materials. Moreover, the influence of O2 dilution in the inlet gas upon the temperature profiles during the Cu oxidation stage was assessed. The recirculation of a large part of the product gas resulting from the oxidation stage moderates the temperature profile and the complete conversion of the Cu-based solids is achieved in a relatively sharp oxidation front despite the low O2 content in the feed (i.e., 3 vol% O2). The experimental results are in close agreement with the predictions of a one-dimensional reactor model that incorporates existing information taken from the literature on the kinetics, fluid dynamics and heat transfer.
57
Journal: Chemical Engineering Journal - Volume 325, 1 October 2017, Pages 208-220