| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 6475214 | 1424967 | 2017 | 13 صفحه PDF | دانلود رایگان |
- Equilibrium concentrations of H2S, COS, HCl and HF are computed for solid sorbents.
- The solid sorbents are based on pure solids: ZnO, cerium oxides, La2O3 and La2O2CO3.
- Interferences of HCl and HF in de-H2S reactions are determined (TÂ =Â 500-1100Â K).
- Minimization of Gibbs free energy for the complex systems is used.
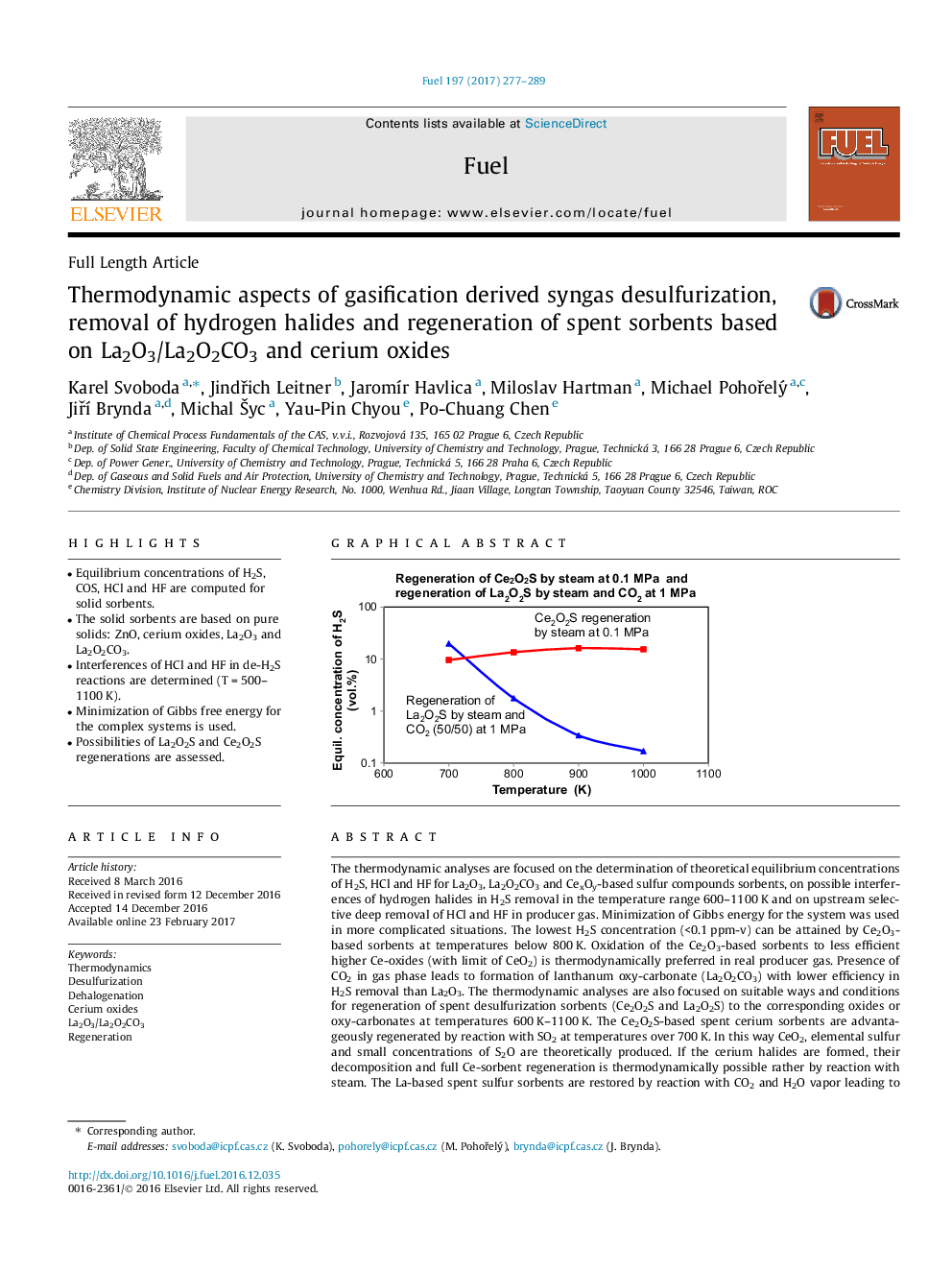
- Possibilities of La2O2S and Ce2O2S regenerations are assessed.
The thermodynamic analyses are focused on the determination of theoretical equilibrium concentrations of H2S, HCl and HF for La2O3, La2O2CO3 and CexOy-based sulfur compounds sorbents, on possible interferences of hydrogen halides in H2S removal in the temperature range 600-1100Â K and on upstream selective deep removal of HCl and HF in producer gas. Minimization of Gibbs energy for the system was used in more complicated situations. The lowest H2S concentration (<0.1Â ppm-v) can be attained by Ce2O3-based sorbents at temperatures below 800Â K. Oxidation of the Ce2O3-based sorbents to less efficient higher Ce-oxides (with limit of CeO2) is thermodynamically preferred in real producer gas. Presence of CO2 in gas phase leads to formation of lanthanum oxy-carbonate (La2O2CO3) with lower efficiency in H2S removal than La2O3. The thermodynamic analyses are also focused on suitable ways and conditions for regeneration of spent desulfurization sorbents (Ce2O2S and La2O2S) to the corresponding oxides or oxy-carbonates at temperatures 600Â K-1100Â K. The Ce2O2S-based spent cerium sorbents are advantageously regenerated by reaction with SO2 at temperatures over 700Â K. In this way CeO2, elemental sulfur and small concentrations of S2O are theoretically produced. If the cerium halides are formed, their decomposition and full Ce-sorbent regeneration is thermodynamically possible rather by reaction with steam. The La-based spent sulfur sorbents are restored by reaction with CO2 and H2O vapor leading to formation of La2O2CO3 and H2S. The suitable operating conditions involve temperatures between 700 and 800Â K and higher operating pressures. Potential small contents of La-oxy-halides (LaOF and LaOCl) in the spent sorbent are converted into La2O2CO3 in such a regeneration process.
161
Journal: Fuel - Volume 197, 1 June 2017, Pages 277-289