| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 6478787 | 1428099 | 2017 | 10 صفحه PDF | دانلود رایگان |
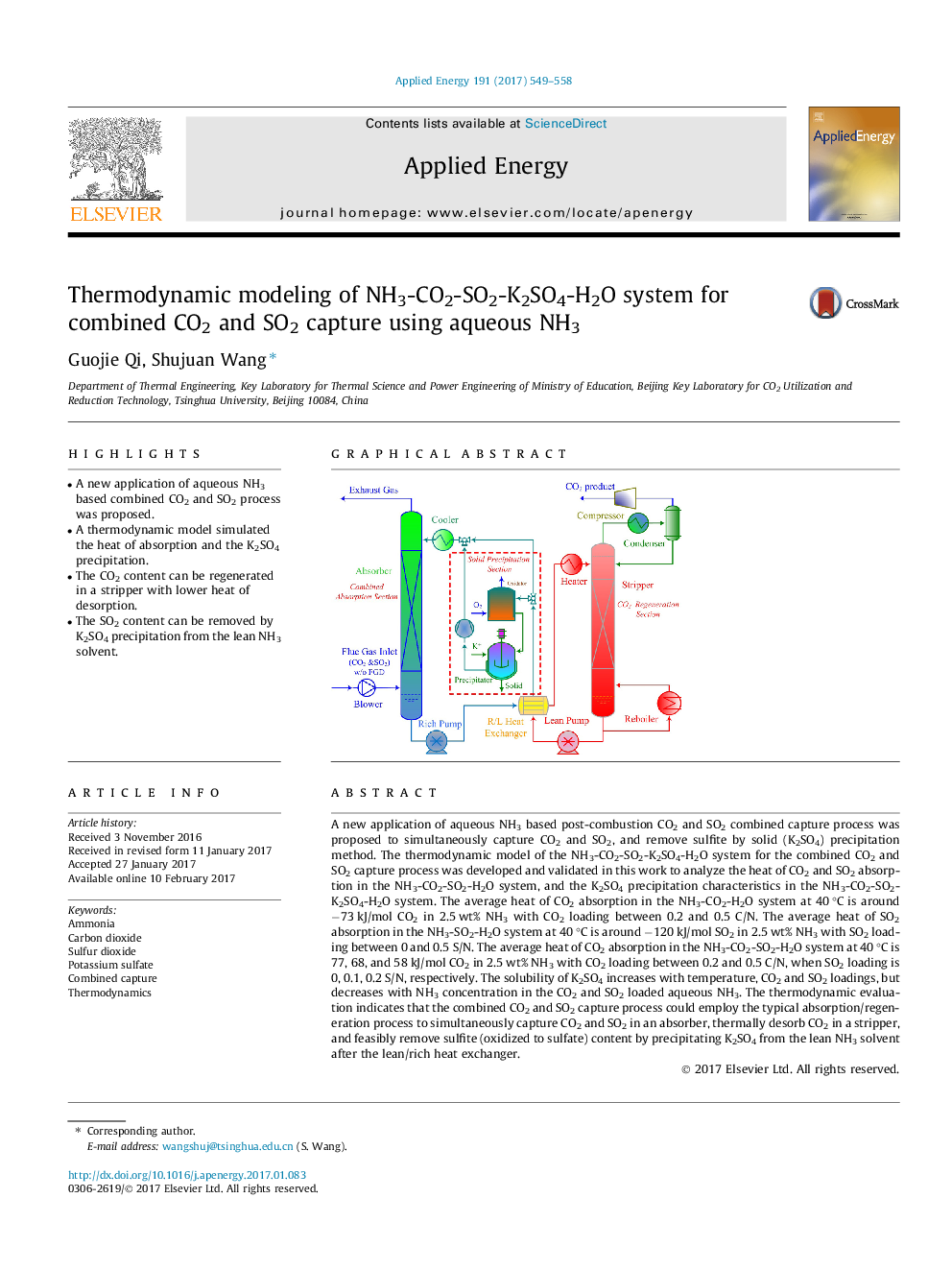
- A new application of aqueous NH3 based combined CO2 and SO2 process was proposed.
- A thermodynamic model simulated the heat of absorption and the K2SO4 precipitation.
- The CO2 content can be regenerated in a stripper with lower heat of desorption.
- The SO2 content can be removed by K2SO4 precipitation from the lean NH3 solvent.
A new application of aqueous NH3 based post-combustion CO2 and SO2 combined capture process was proposed to simultaneously capture CO2 and SO2, and remove sulfite by solid (K2SO4) precipitation method. The thermodynamic model of the NH3-CO2-SO2-K2SO4-H2O system for the combined CO2 and SO2 capture process was developed and validated in this work to analyze the heat of CO2 and SO2 absorption in the NH3-CO2-SO2-H2O system, and the K2SO4 precipitation characteristics in the NH3-CO2-SO2-K2SO4-H2O system. The average heat of CO2 absorption in the NH3-CO2-H2O system at 40 °C is around â73 kJ/mol CO2 in 2.5 wt% NH3 with CO2 loading between 0.2 and 0.5 C/N. The average heat of SO2 absorption in the NH3-SO2-H2O system at 40 °C is around â120 kJ/mol SO2 in 2.5 wt% NH3 with SO2 loading between 0 and 0.5 S/N. The average heat of CO2 absorption in the NH3-CO2-SO2-H2O system at 40 °C is 77, 68, and 58 kJ/mol CO2 in 2.5 wt% NH3 with CO2 loading between 0.2 and 0.5 C/N, when SO2 loading is 0, 0.1, 0.2 S/N, respectively. The solubility of K2SO4 increases with temperature, CO2 and SO2 loadings, but decreases with NH3 concentration in the CO2 and SO2 loaded aqueous NH3. The thermodynamic evaluation indicates that the combined CO2 and SO2 capture process could employ the typical absorption/regeneration process to simultaneously capture CO2 and SO2 in an absorber, thermally desorb CO2 in a stripper, and feasibly remove sulfite (oxidized to sulfate) content by precipitating K2SO4 from the lean NH3 solvent after the lean/rich heat exchanger.
167
Journal: Applied Energy - Volume 191, 1 April 2017, Pages 549-558