| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1228969 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 10 Pages |
•Nanocomposites TiO2–SiO2 thin films were prepared by sol–gel dip-coating process.•The composition-dependent structural and optical properties were examined.•The refractive index dispersion fit well the Wemple-DiDomenico single oscillator model.•The optical parameters were tunable by a simple control on the film composition.
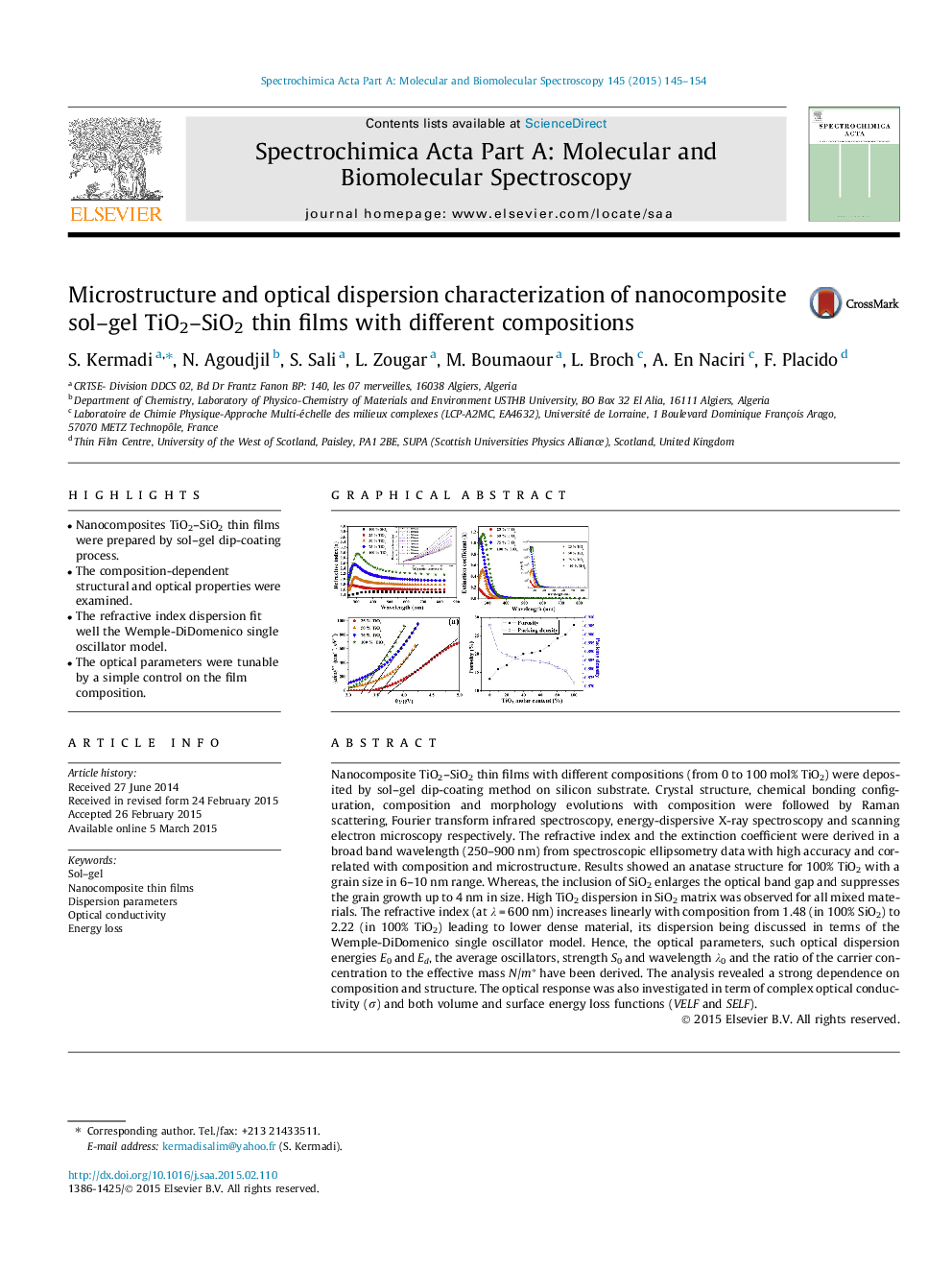
Nanocomposite TiO2–SiO2 thin films with different compositions (from 0 to 100 mol% TiO2) were deposited by sol–gel dip-coating method on silicon substrate. Crystal structure, chemical bonding configuration, composition and morphology evolutions with composition were followed by Raman scattering, Fourier transform infrared spectroscopy, energy-dispersive X-ray spectroscopy and scanning electron microscopy respectively. The refractive index and the extinction coefficient were derived in a broad band wavelength (250–900 nm) from spectroscopic ellipsometry data with high accuracy and correlated with composition and microstructure. Results showed an anatase structure for 100% TiO2 with a grain size in 6–10 nm range. Whereas, the inclusion of SiO2 enlarges the optical band gap and suppresses the grain growth up to 4 nm in size. High TiO2 dispersion in SiO2 matrix was observed for all mixed materials. The refractive index (at λ = 600 nm) increases linearly with composition from 1.48 (in 100% SiO2) to 2.22 (in 100% TiO2) leading to lower dense material, its dispersion being discussed in terms of the Wemple-DiDomenico single oscillator model. Hence, the optical parameters, such optical dispersion energies E0 and Ed, the average oscillators, strength S0 and wavelength λ0 and the ratio of the carrier concentration to the effective mass N/m∗ have been derived. The analysis revealed a strong dependence on composition and structure. The optical response was also investigated in term of complex optical conductivity (σ) and both volume and surface energy loss functions (VELF and SELF).
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide