| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1228973 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 7 Pages |
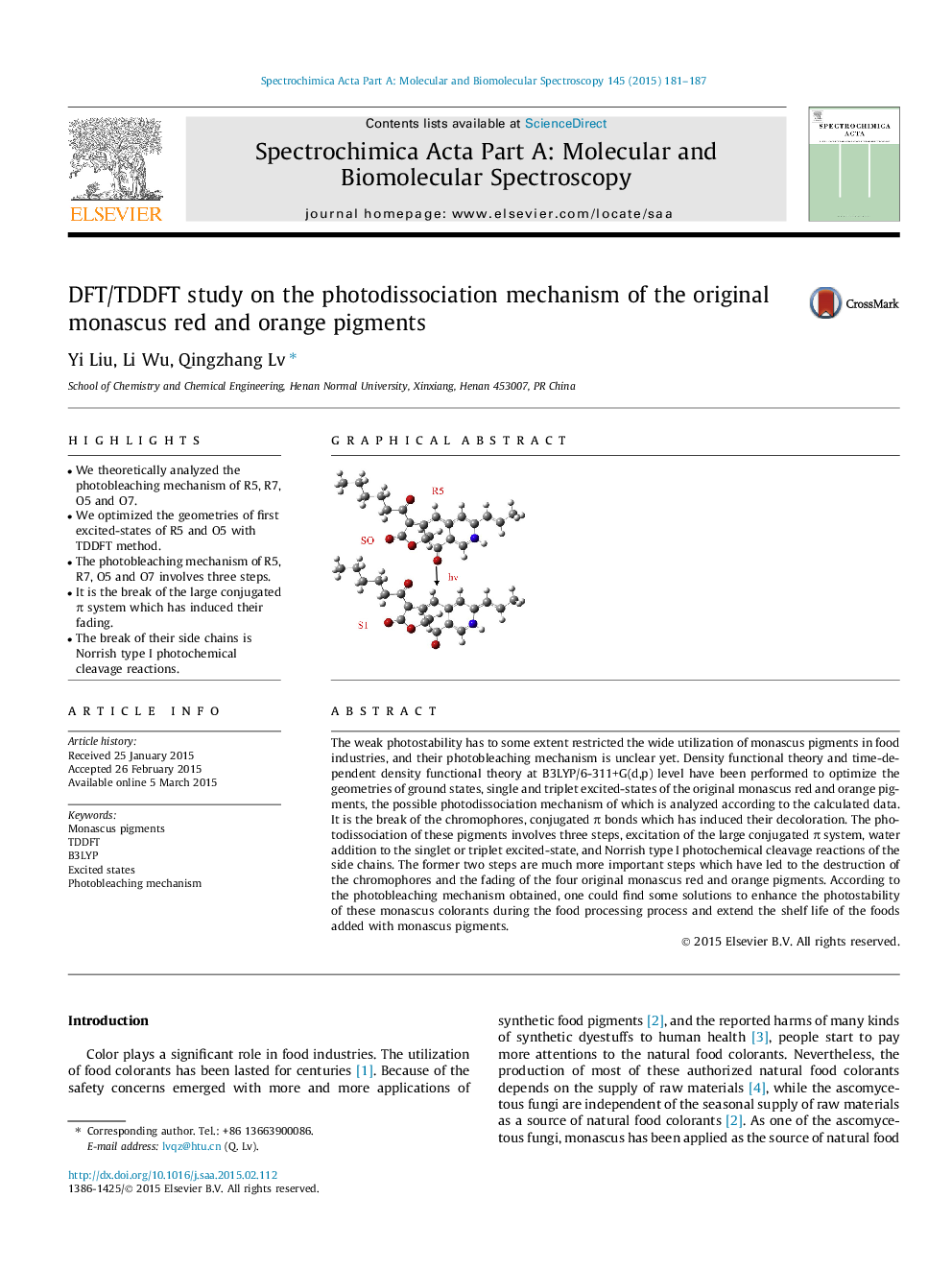
•We theoretically analyzed the photobleaching mechanism of R5, R7, O5 and O7.•We optimized the geometries of first excited-states of R5 and O5 with TDDFT method.•The photobleaching mechanism of R5, R7, O5 and O7 involves three steps.•It is the break of the large conjugated π system which has induced their fading.•The break of their side chains is Norrish type I photochemical cleavage reactions.
The weak photostability has to some extent restricted the wide utilization of monascus pigments in food industries, and their photobleaching mechanism is unclear yet. Density functional theory and time-dependent density functional theory at B3LYP/6-311+G(d,p) level have been performed to optimize the geometries of ground states, single and triplet excited-states of the original monascus red and orange pigments, the possible photodissociation mechanism of which is analyzed according to the calculated data. It is the break of the chromophores, conjugated π bonds which has induced their decoloration. The photodissociation of these pigments involves three steps, excitation of the large conjugated π system, water addition to the singlet or triplet excited-state, and Norrish type I photochemical cleavage reactions of the side chains. The former two steps are much more important steps which have led to the destruction of the chromophores and the fading of the four original monascus red and orange pigments. According to the photobleaching mechanism obtained, one could find some solutions to enhance the photostability of these monascus colorants during the food processing process and extend the shelf life of the foods added with monascus pigments.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide