| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229017 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 13 Pages |
•Experimental results indicated that rac-VEN interact with DNA as a groove binder.•Hydrogen bond and van der Waals force play main roles in the binding.•The S isomer of VEN has made better interactions with the B-DNA than R isomer.
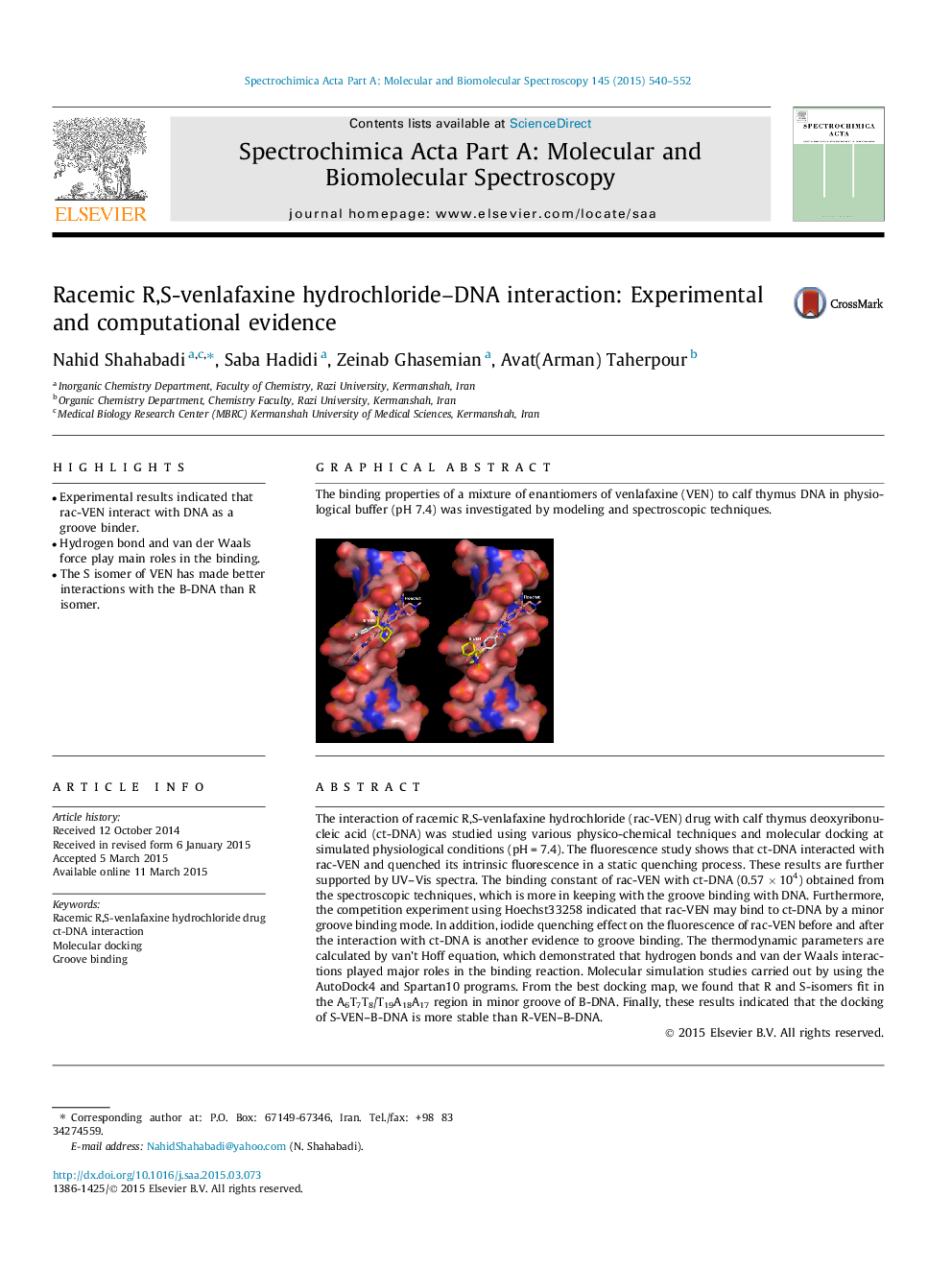
The interaction of racemic R,S-venlafaxine hydrochloride (rac-VEN) drug with calf thymus deoxyribonucleic acid (ct-DNA) was studied using various physico-chemical techniques and molecular docking at simulated physiological conditions (pH = 7.4). The fluorescence study shows that ct-DNA interacted with rac-VEN and quenched its intrinsic fluorescence in a static quenching process. These results are further supported by UV–Vis spectra. The binding constant of rac-VEN with ct-DNA (0.57 × 104) obtained from the spectroscopic techniques, which is more in keeping with the groove binding with DNA. Furthermore, the competition experiment using Hoechst33258 indicated that rac-VEN may bind to ct-DNA by a minor groove binding mode. In addition, iodide quenching effect on the fluorescence of rac-VEN before and after the interaction with ct-DNA is another evidence to groove binding. The thermodynamic parameters are calculated by van’t Hoff equation, which demonstrated that hydrogen bonds and van der Waals interactions played major roles in the binding reaction. Molecular simulation studies carried out by using the AutoDock4 and Spartan10 programs. From the best docking map, we found that R and S-isomers fit in the A6T7T8/T19A18A17 region in minor groove of B-DNA. Finally, these results indicated that the docking of S-VEN–B-DNA is more stable than R-VEN–B-DNA.
Graphical abstractThe binding properties of a mixture of enantiomers of venlafaxine (VEN) to calf thymus DNA in physiological buffer (pH 7.4) was investigated by modeling and spectroscopic techniques.Figure optionsDownload full-size imageDownload as PowerPoint slide