| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229030 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 12 Pages |
•Spectroscopic analyses of BEPA were examined by FT-IR, FT-Raman, UV–Vis and NMR techniques.•Molecular structural parameters of the geometry have been computed by B3LYP and M06-2X methods.•The wavenumbers are assigned using PED analysis.•FMO and NBO analyses were also performed.•Molecular electrostatic potential of the title compound was calculated.
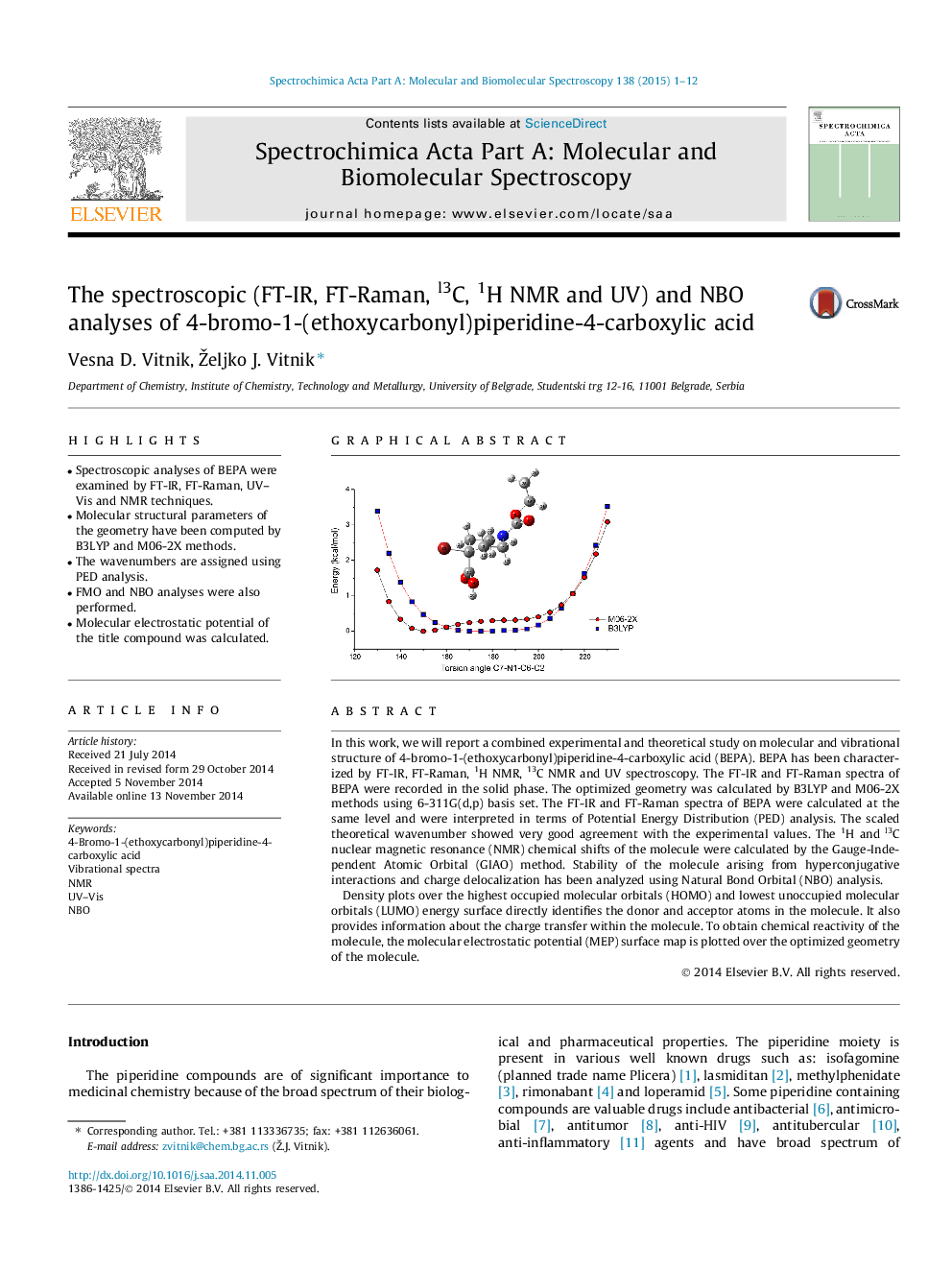
In this work, we will report a combined experimental and theoretical study on molecular and vibrational structure of 4-bromo-1-(ethoxycarbonyl)piperidine-4-carboxylic acid (BEPA). BEPA has been characterized by FT-IR, FT-Raman, 1H NMR, 13C NMR and UV spectroscopy. The FT-IR and FT-Raman spectra of BEPA were recorded in the solid phase. The optimized geometry was calculated by B3LYP and M06-2X methods using 6-311G(d,p) basis set. The FT-IR and FT-Raman spectra of BEPA were calculated at the same level and were interpreted in terms of Potential Energy Distribution (PED) analysis. The scaled theoretical wavenumber showed very good agreement with the experimental values. The 1H and l3C nuclear magnetic resonance (NMR) chemical shifts of the molecule were calculated by the Gauge-Independent Atomic Orbital (GIAO) method. Stability of the molecule arising from hyperconjugative interactions and charge delocalization has been analyzed using Natural Bond Orbital (NBO) analysis.Density plots over the highest occupied molecular orbitals (HOMO) and lowest unoccupied molecular orbitals (LUMO) energy surface directly identifies the donor and acceptor atoms in the molecule. It also provides information about the charge transfer within the molecule. To obtain chemical reactivity of the molecule, the molecular electrostatic potential (MEP) surface map is plotted over the optimized geometry of the molecule.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide