| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229033 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 7 Pages |
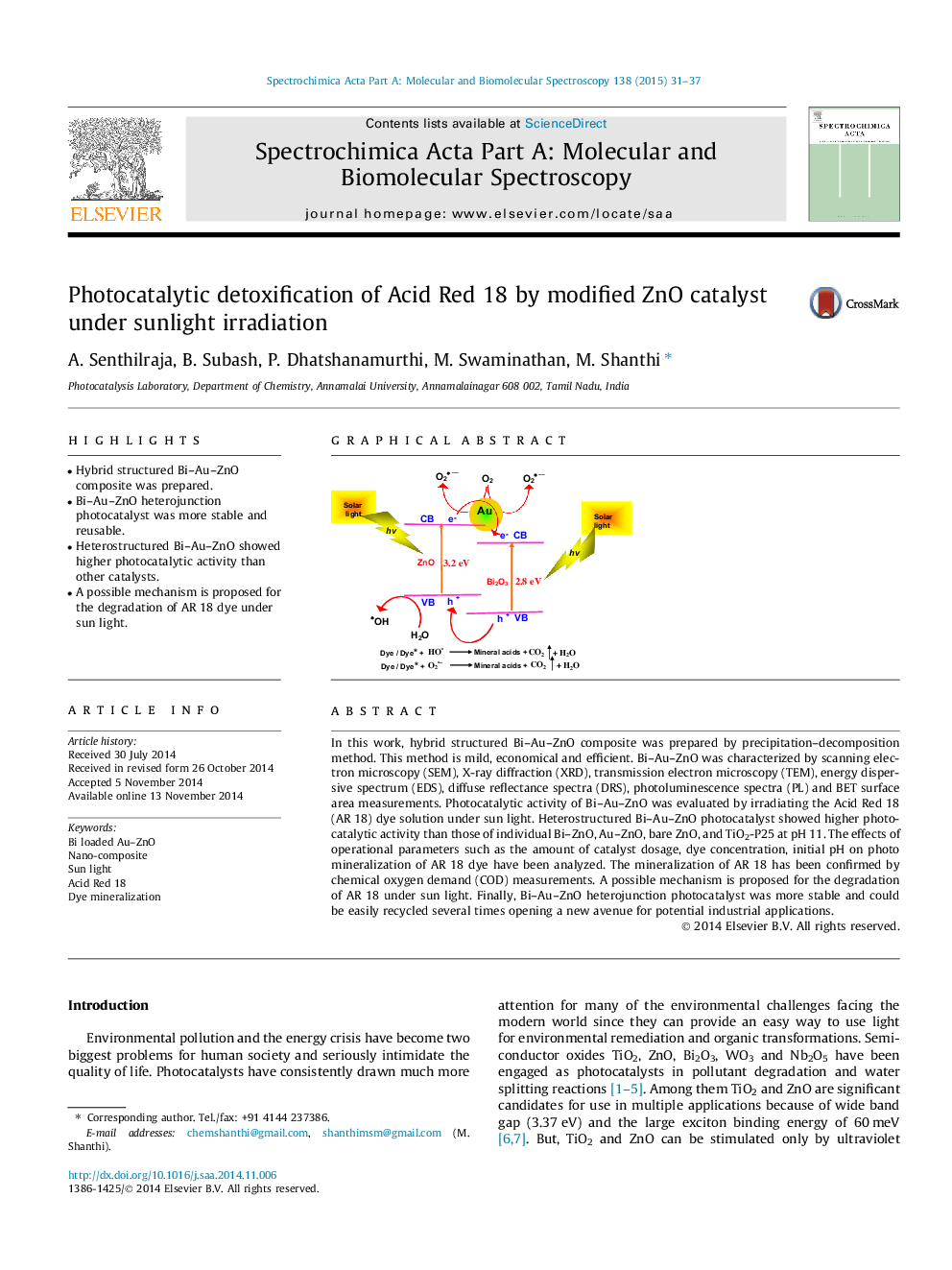
•Hybrid structured Bi–Au–ZnO composite was prepared.•Bi–Au–ZnO heterojunction photocatalyst was more stable and reusable.•Heterostructured Bi–Au–ZnO showed higher photocatalytic activity than other catalysts.•A possible mechanism is proposed for the degradation of AR 18 dye under sun light.
In this work, hybrid structured Bi–Au–ZnO composite was prepared by precipitation–decomposition method. This method is mild, economical and efficient. Bi–Au–ZnO was characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD), transmission electron microscopy (TEM), energy dispersive spectrum (EDS), diffuse reflectance spectra (DRS), photoluminescence spectra (PL) and BET surface area measurements. Photocatalytic activity of Bi–Au–ZnO was evaluated by irradiating the Acid Red 18 (AR 18) dye solution under sun light. Heterostructured Bi–Au–ZnO photocatalyst showed higher photocatalytic activity than those of individual Bi–ZnO, Au–ZnO, bare ZnO, and TiO2-P25 at pH 11. The effects of operational parameters such as the amount of catalyst dosage, dye concentration, initial pH on photo mineralization of AR 18 dye have been analyzed. The mineralization of AR 18 has been confirmed by chemical oxygen demand (COD) measurements. A possible mechanism is proposed for the degradation of AR 18 under sun light. Finally, Bi–Au–ZnO heterojunction photocatalyst was more stable and could be easily recycled several times opening a new avenue for potential industrial applications.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide