| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229039 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 7 Pages |
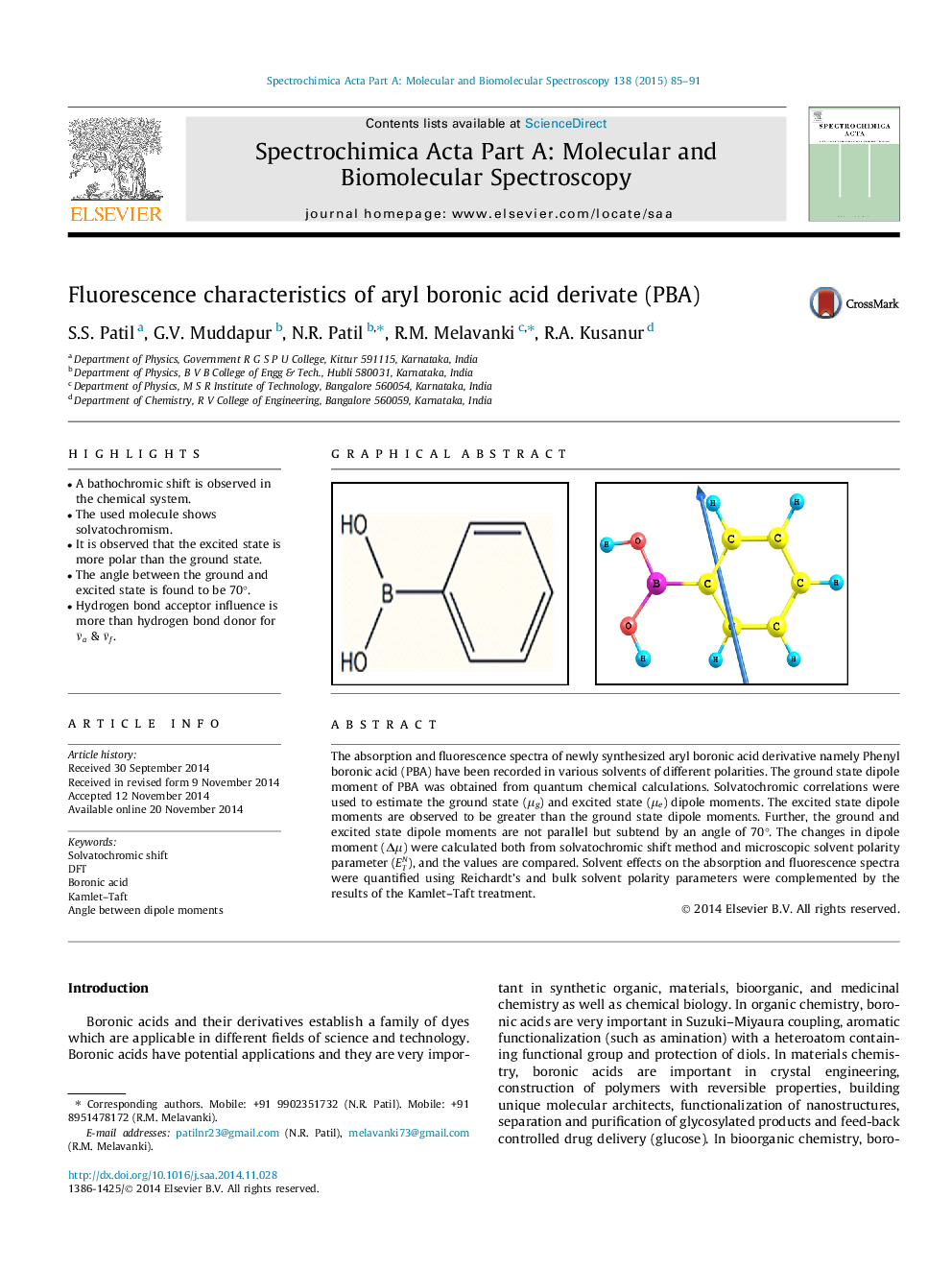
•A bathochromic shift is observed in the chemical system.•The used molecule shows solvatochromism.•It is observed that the excited state is more polar than the ground state.•The angle between the ground and excited state is found to be 70°.•Hydrogen bond acceptor influence is more than hydrogen bond donor for ν¯a & ν¯f.
The absorption and fluorescence spectra of newly synthesized aryl boronic acid derivative namely Phenyl boronic acid (PBA) have been recorded in various solvents of different polarities. The ground state dipole moment of PBA was obtained from quantum chemical calculations. Solvatochromic correlations were used to estimate the ground state (μg) and excited state (μe) dipole moments. The excited state dipole moments are observed to be greater than the ground state dipole moments. Further, the ground and excited state dipole moments are not parallel but subtend by an angle of 70°. The changes in dipole moment (Δμ ) were calculated both from solvatochromic shift method and microscopic solvent polarity parameter (ETN), and the values are compared. Solvent effects on the absorption and fluorescence spectra were quantified using Reichardt’s and bulk solvent polarity parameters were complemented by the results of the Kamlet–Taft treatment.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide