| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229056 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 4 Pages |
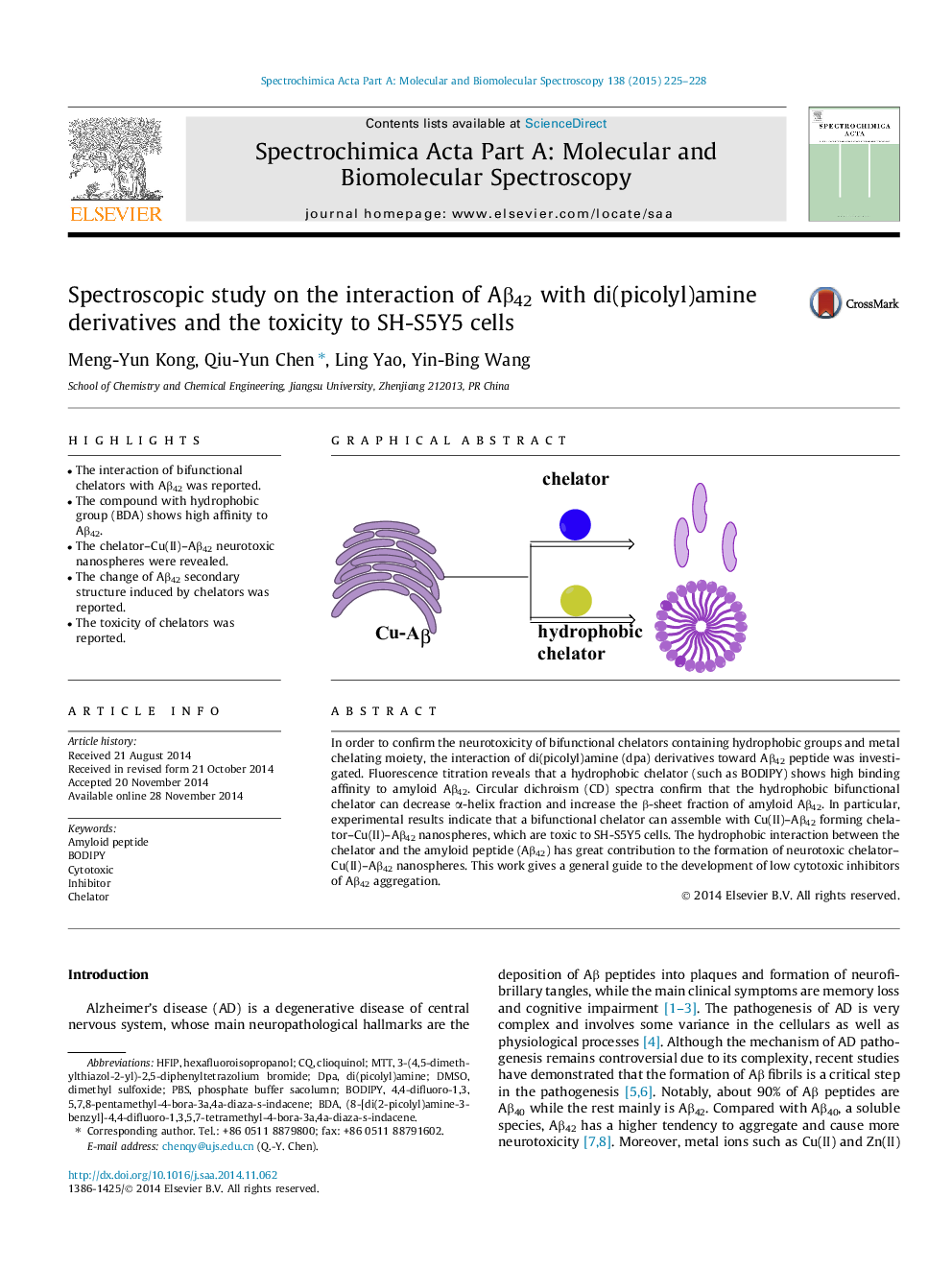
•The interaction of bifunctional chelators with Aβ42 was reported.•The compound with hydrophobic group (BDA) shows high affinity to Aβ42.•The chelator–Cu(II)–Aβ42 neurotoxic nanospheres were revealed.•The change of Aβ42 secondary structure induced by chelators was reported.•The toxicity of chelators was reported.
In order to confirm the neurotoxicity of bifunctional chelators containing hydrophobic groups and metal chelating moiety, the interaction of di(picolyl)amine (dpa) derivatives toward Aβ42 peptide was investigated. Fluorescence titration reveals that a hydrophobic chelator (such as BODIPY) shows high binding affinity to amyloid Aβ42. Circular dichroism (CD) spectra confirm that the hydrophobic bifunctional chelator can decrease α-helix fraction and increase the β-sheet fraction of amyloid Aβ42. In particular, experimental results indicate that a bifunctional chelator can assemble with Cu(II)–Aβ42 forming chelator–Cu(II)–Aβ42 nanospheres, which are toxic to SH-S5Y5 cells. The hydrophobic interaction between the chelator and the amyloid peptide (Aβ42) has great contribution to the formation of neurotoxic chelator–Cu(II)–Aβ42 nanospheres. This work gives a general guide to the development of low cytotoxic inhibitors of Aβ42 aggregation.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide