| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229067 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 7 Pages |
•TiO2 supported on CNSAC exhibits porous morphology.•In the synthesized composite catalyst, TiO2 exists in anatase phase.•Average crystallite size of TiO2 reduces upon loading on CNSAC.•Reduction in crystallite size gives rise to more photoexcitation and adsorption sites.
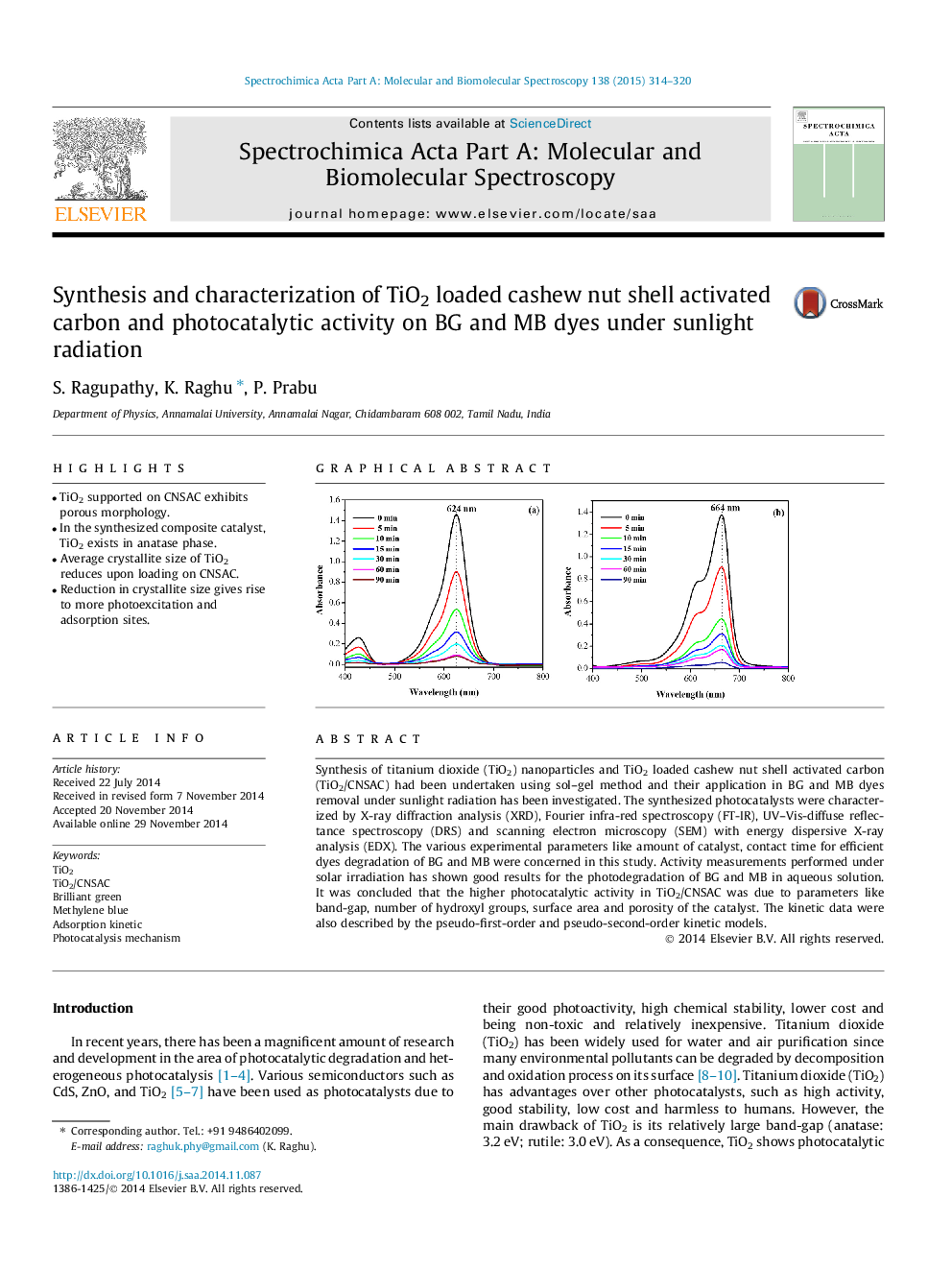
Synthesis of titanium dioxide (TiO2) nanoparticles and TiO2 loaded cashew nut shell activated carbon (TiO2/CNSAC) had been undertaken using sol–gel method and their application in BG and MB dyes removal under sunlight radiation has been investigated. The synthesized photocatalysts were characterized by X-ray diffraction analysis (XRD), Fourier infra-red spectroscopy (FT-IR), UV–Vis-diffuse reflectance spectroscopy (DRS) and scanning electron microscopy (SEM) with energy dispersive X-ray analysis (EDX). The various experimental parameters like amount of catalyst, contact time for efficient dyes degradation of BG and MB were concerned in this study. Activity measurements performed under solar irradiation has shown good results for the photodegradation of BG and MB in aqueous solution. It was concluded that the higher photocatalytic activity in TiO2/CNSAC was due to parameters like band-gap, number of hydroxyl groups, surface area and porosity of the catalyst. The kinetic data were also described by the pseudo-first-order and pseudo-second-order kinetic models.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide