| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229079 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 10 Pages |
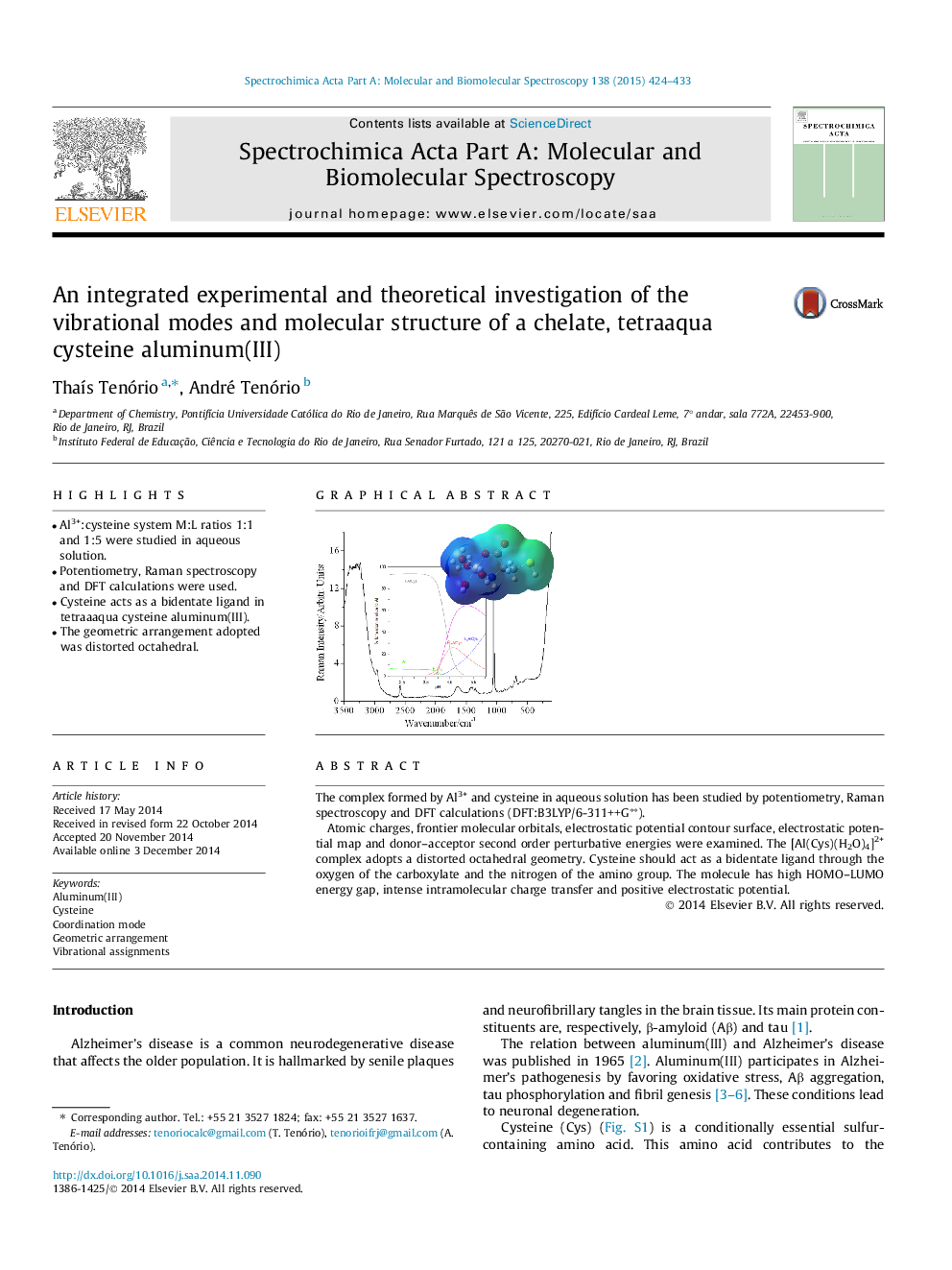
•Al3+:cysteine system M:L ratios 1:1 and 1:5 were studied in aqueous solution.•Potentiometry, Raman spectroscopy and DFT calculations were used.•Cysteine acts as a bidentate ligand in tetraaaqua cysteine aluminum(III).•The geometric arrangement adopted was distorted octahedral.
The complex formed by Al3+ and cysteine in aqueous solution has been studied by potentiometry, Raman spectroscopy and DFT calculations (DFT:B3LYP/6-311++G∗∗).Atomic charges, frontier molecular orbitals, electrostatic potential contour surface, electrostatic potential map and donor–acceptor second order perturbative energies were examined. The [Al(Cys)(H2O)4]2+ complex adopts a distorted octahedral geometry. Cysteine should act as a bidentate ligand through the oxygen of the carboxylate and the nitrogen of the amino group. The molecule has high HOMO–LUMO energy gap, intense intramolecular charge transfer and positive electrostatic potential.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide