| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229089 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 12 Pages |
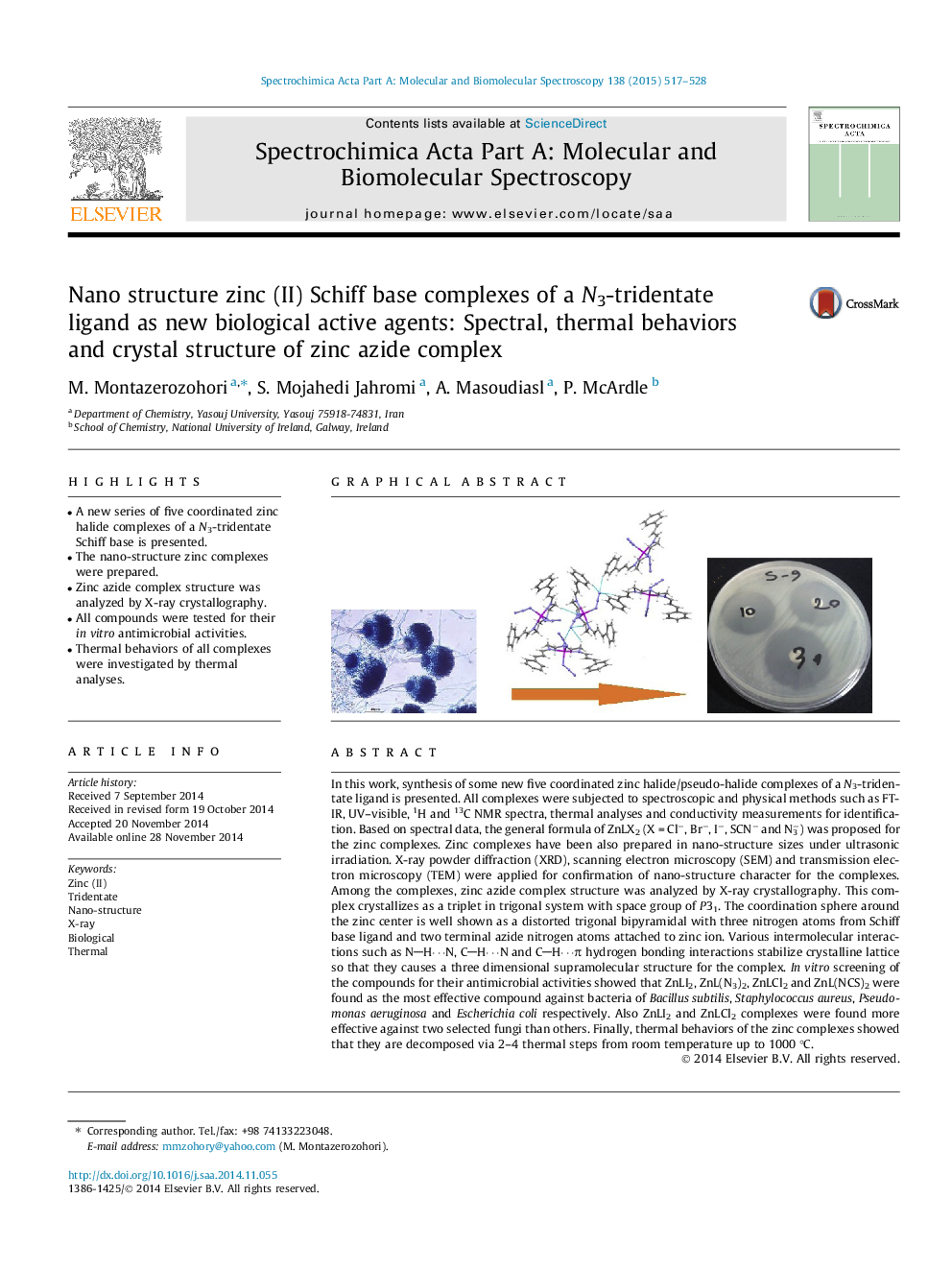
•A new series of five coordinated zinc halide complexes of a N3-tridentate Schiff base is presented.•The nano-structure zinc complexes were prepared.•Zinc azide complex structure was analyzed by X-ray crystallography.•All compounds were tested for their in vitro antimicrobial activities.•Thermal behaviors of all complexes were investigated by thermal analyses.
In this work, synthesis of some new five coordinated zinc halide/pseudo-halide complexes of a N3-tridentate ligand is presented. All complexes were subjected to spectroscopic and physical methods such as FT-IR, UV–visible, 1H and 13C NMR spectra, thermal analyses and conductivity measurements for identification. Based on spectral data, the general formula of ZnLX2 (X = Cl−, Br−, I−, SCN− and N3−) was proposed for the zinc complexes. Zinc complexes have been also prepared in nano-structure sizes under ultrasonic irradiation. X-ray powder diffraction (XRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were applied for confirmation of nano-structure character for the complexes. Among the complexes, zinc azide complex structure was analyzed by X-ray crystallography. This complex crystallizes as a triplet in trigonal system with space group of P31. The coordination sphere around the zinc center is well shown as a distorted trigonal bipyramidal with three nitrogen atoms from Schiff base ligand and two terminal azide nitrogen atoms attached to zinc ion. Various intermolecular interactions such as NH⋯N, CH⋯N and CH⋯π hydrogen bonding interactions stabilize crystalline lattice so that they causes a three dimensional supramolecular structure for the complex. In vitro screening of the compounds for their antimicrobial activities showed that ZnLI2, ZnL(N3)2, ZnLCl2 and ZnL(NCS)2 were found as the most effective compound against bacteria of Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa and Escherichia coli respectively. Also ZnLI2 and ZnLCl2 complexes were found more effective against two selected fungi than others. Finally, thermal behaviors of the zinc complexes showed that they are decomposed via 2–4 thermal steps from room temperature up to 1000 °C.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide