| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229102 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 5 Pages |
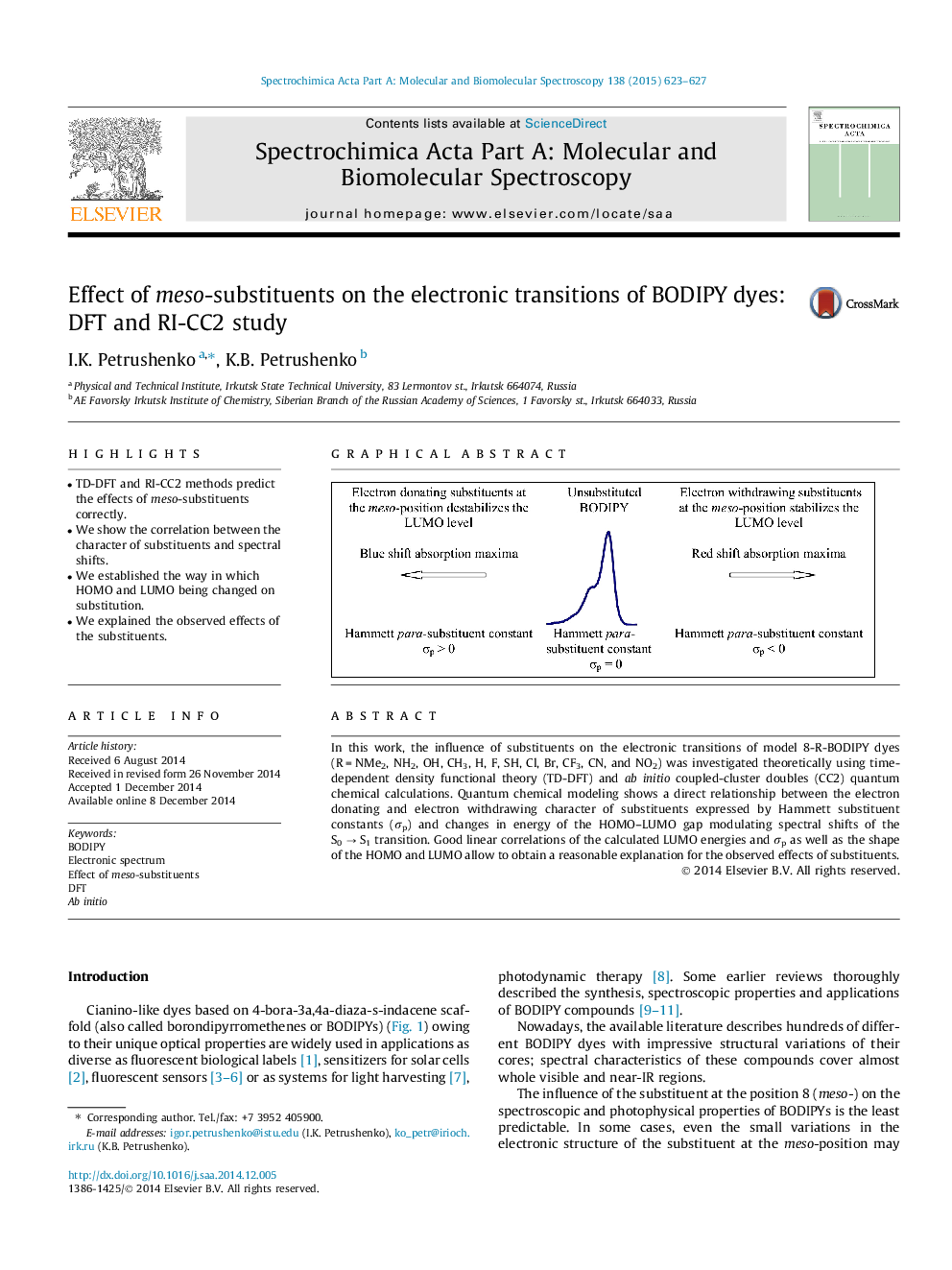
•TD-DFT and RI-CC2 methods predict the effects of meso-substituents correctly.•We show the correlation between the character of substituents and spectral shifts.•We established the way in which HOMO and LUMO being changed on substitution.•We explained the observed effects of the substituents.
In this work, the influence of substituents on the electronic transitions of model 8-R-BODIPY dyes (R = NMe2, NH2, OH, CH3, H, F, SH, Cl, Br, CF3, CN, and NO2) was investigated theoretically using time-dependent density functional theory (TD-DFT) and ab initio coupled-cluster doubles (CC2) quantum chemical calculations. Quantum chemical modeling shows a direct relationship between the electron donating and electron withdrawing character of substituents expressed by Hammett substituent constants (σp) and changes in energy of the HOMO–LUMO gap modulating spectral shifts of the S0 → S1 transition. Good linear correlations of the calculated LUMO energies and σp as well as the shape of the HOMO and LUMO allow to obtain a reasonable explanation for the observed effects of substituents.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide