| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229104 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 6 Pages |
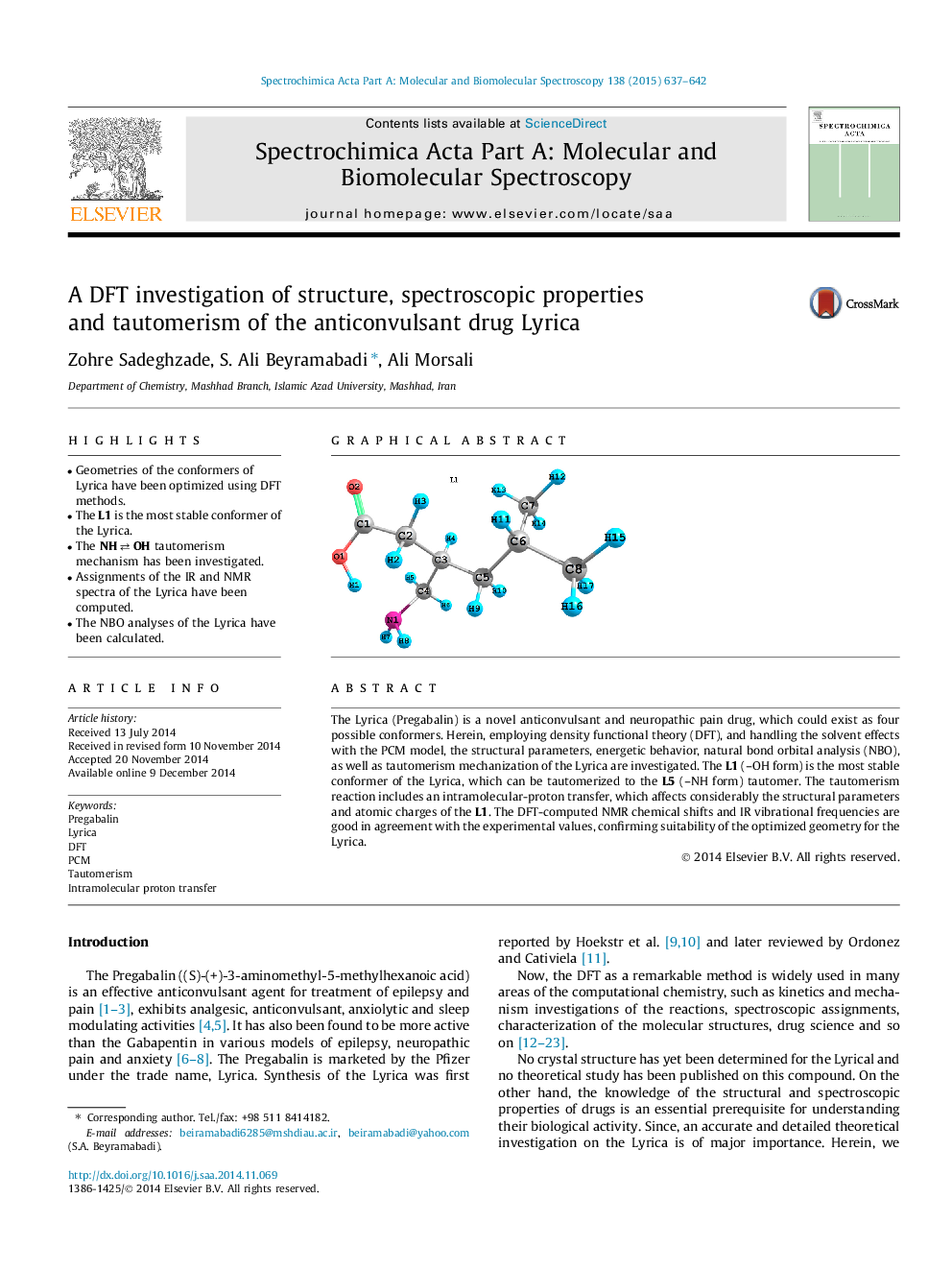
•Geometries of the conformers of Lyrica have been optimized using DFT methods.•The L1 is the most stable conformer of the Lyrica.•The NH⇄OHNH⇄OH tautomerism mechanism has been investigated.•Assignments of the IR and NMR spectra of the Lyrica have been computed.•The NBO analyses of the Lyrica have been calculated.
The Lyrica (Pregabalin) is a novel anticonvulsant and neuropathic pain drug, which could exist as four possible conformers. Herein, employing density functional theory (DFT), and handling the solvent effects with the PCM model, the structural parameters, energetic behavior, natural bond orbital analysis (NBO), as well as tautomerism mechanization of the Lyrica are investigated. The L1 (–OH form) is the most stable conformer of the Lyrica, which can be tautomerized to the L5 (–NH form) tautomer. The tautomerism reaction includes an intramolecular-proton transfer, which affects considerably the structural parameters and atomic charges of the L1. The DFT-computed NMR chemical shifts and IR vibrational frequencies are good in agreement with the experimental values, confirming suitability of the optimized geometry for the Lyrica.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide