| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229105 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 5 Pages |
•Azo-azomethine dyes with triazine based.•The tautomerism between enol-amine, enaminone and hydrazone forms in organic solvent was studied.•The solvatochromism is dependent on the substitution, solvent, pH and temperature.•The azo-azomethine dyes has antioxidant activity, which are dependent on electron releasing of substituents.
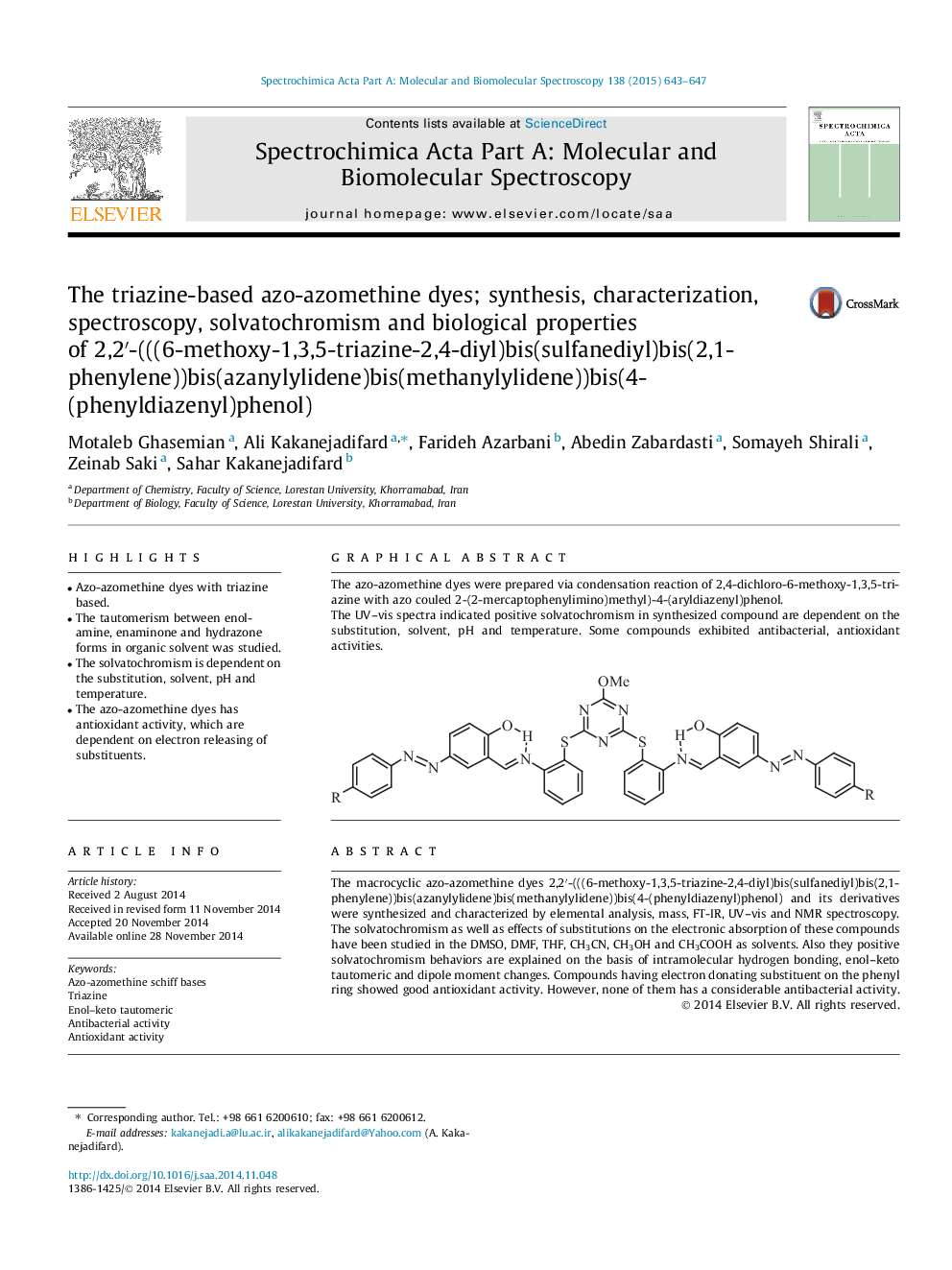
The macrocyclic azo-azomethine dyes 2,2′-(((6-methoxy-1,3,5-triazine-2,4-diyl)bis(sulfanediyl)bis(2,1-phenylene))bis(azanylylidene)bis(methanylylidene))bis(4-(phenyldiazenyl)phenol) and its derivatives were synthesized and characterized by elemental analysis, mass, FT-IR, UV–vis and NMR spectroscopy. The solvatochromism as well as effects of substitutions on the electronic absorption of these compounds have been studied in the DMSO, DMF, THF, CH3CN, CH3OH and CH3COOH as solvents. Also they positive solvatochromism behaviors are explained on the basis of intramolecular hydrogen bonding, enol–keto tautomeric and dipole moment changes. Compounds having electron donating substituent on the phenyl ring showed good antioxidant activity. However, none of them has a considerable antibacterial activity.
Graphical abstractThe azo-azomethine dyes were prepared via condensation reaction of 2,4-dichloro-6-methoxy-1,3,5-triazine with azo couled 2-(2-mercaptophenylimino)methyl)-4-(aryldiazenyl)phenol.The UV–vis spectra indicated positive solvatochromism in synthesized compound are dependent on the substitution, solvent, pH and temperature. Some compounds exhibited antibacterial, antioxidant activities.Figure optionsDownload full-size imageDownload as PowerPoint slide