| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229153 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 9 Pages |
•Medium effect on self-oxidation/dissociation of QAPs.•Solvent effect on stability of CTAP and TBAP.•Tighter ion-pairing in CTAP due to better influence of polarity parameters.•Better influence of solvent dielectric constant on the reactivity of substrates.•Influence of polarity parameters on the absorption spectra of CTAP and TBAP.
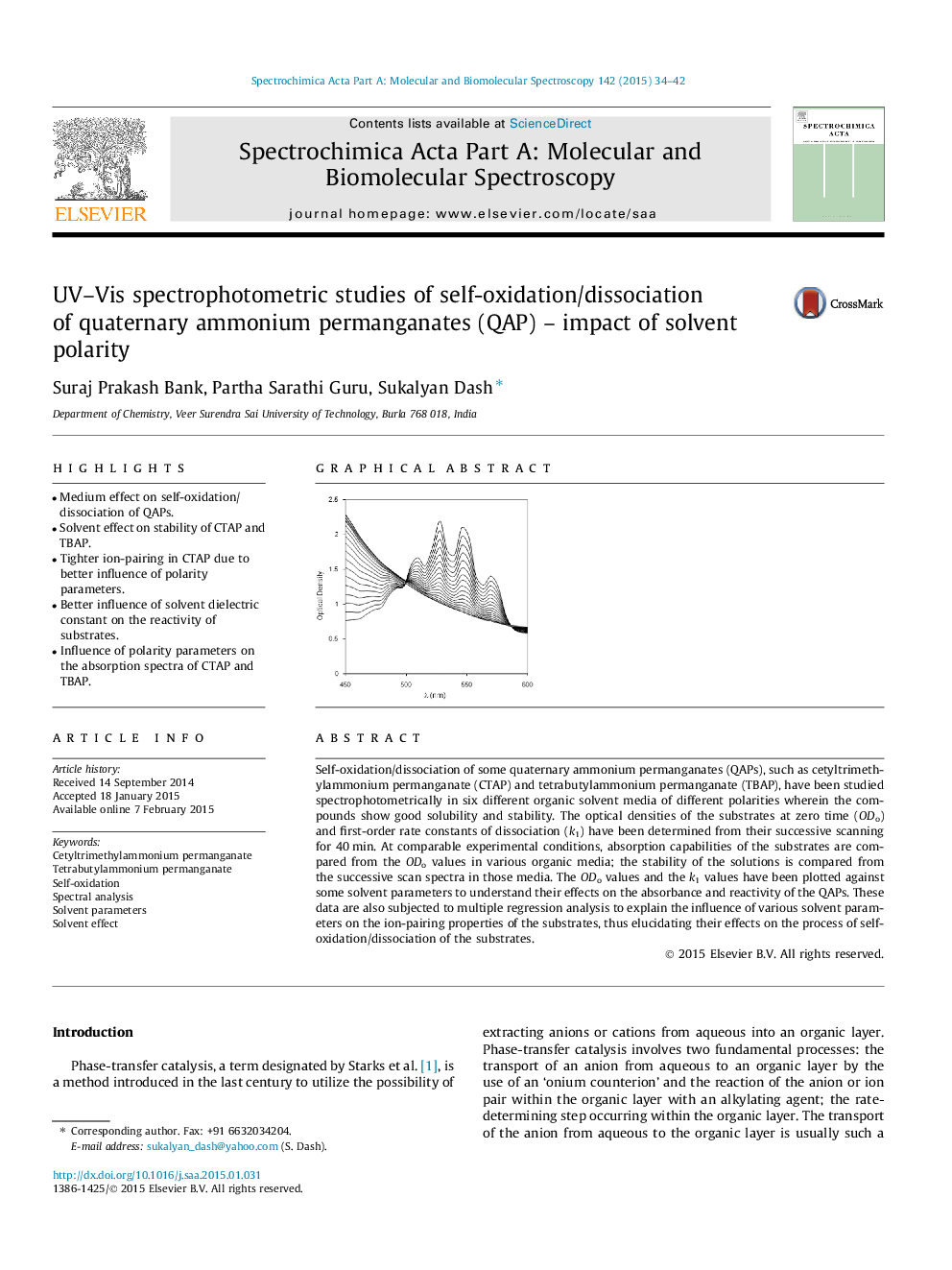
Self-oxidation/dissociation of some quaternary ammonium permanganates (QAPs), such as cetyltrimethylammonium permanganate (CTAP) and tetrabutylammonium permanganate (TBAP), have been studied spectrophotometrically in six different organic solvent media of different polarities wherein the compounds show good solubility and stability. The optical densities of the substrates at zero time (ODo) and first-order rate constants of dissociation (k1) have been determined from their successive scanning for 40 min. At comparable experimental conditions, absorption capabilities of the substrates are compared from the ODo values in various organic media; the stability of the solutions is compared from the successive scan spectra in those media. The ODo values and the k1 values have been plotted against some solvent parameters to understand their effects on the absorbance and reactivity of the QAPs. These data are also subjected to multiple regression analysis to explain the influence of various solvent parameters on the ion-pairing properties of the substrates, thus elucidating their effects on the process of self-oxidation/dissociation of the substrates.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide