| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229165 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 9 Pages |
•The IR spectra of SAC isotopomers were quantitatively analyzed.•The origin of abnormal IR spectral properties of SAC crystals was explained.•The hyperconjugation electronic effects in the IR spectra were recognized.
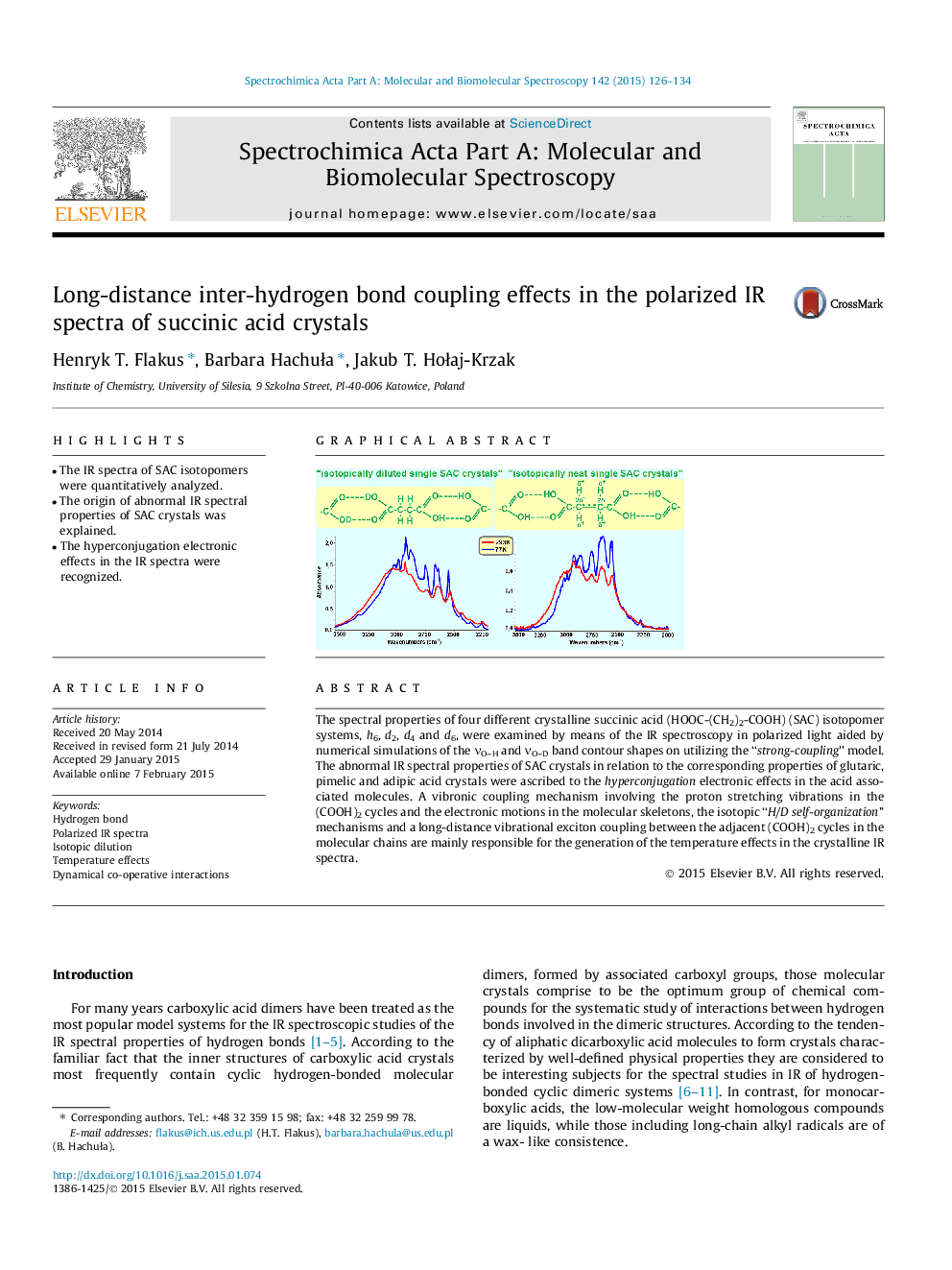
The spectral properties of four different crystalline succinic acid (HOOC-(CH2)2-COOH) (SAC) isotopomer systems, h6, d2, d4 and d6, were examined by means of the IR spectroscopy in polarized light aided by numerical simulations of the νO–H and νO–D band contour shapes on utilizing the “strong-coupling” model. The abnormal IR spectral properties of SAC crystals in relation to the corresponding properties of glutaric, pimelic and adipic acid crystals were ascribed to the hyperconjugation electronic effects in the acid associated molecules. A vibronic coupling mechanism involving the proton stretching vibrations in the (COOH)2 cycles and the electronic motions in the molecular skeletons, the isotopic “H/D self-organization” mechanisms and a long-distance vibrational exciton coupling between the adjacent (COOH)2 cycles in the molecular chains are mainly responsible for the generation of the temperature effects in the crystalline IR spectra.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide