| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229199 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 13 Pages |
•A series of ternary mixed ligand Cu(II) complexes were synthesized and characterized.•The structures of Cu(II) complexes were determined by single crystal X-ray diffraction.•Investigation of DNA–complex interactions was performed by several experimental methods.•The antimicrobial activities of the compounds were evaluated against microorganisms.
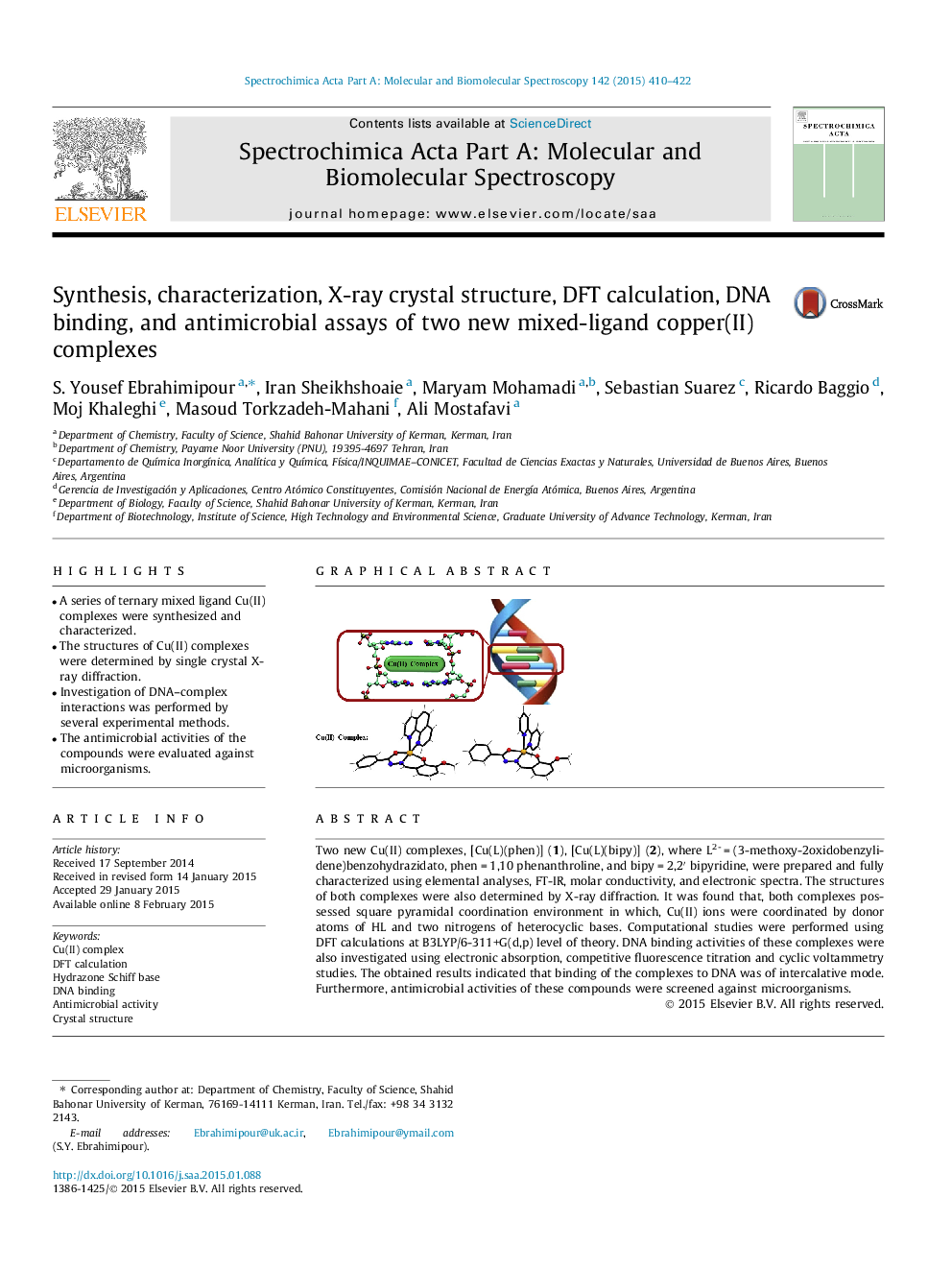
Two new Cu(II) complexes, [Cu(L)(phen)] (1), [Cu(L)(bipy)] (2), where L2- = (3-methoxy-2oxidobenzylidene)benzohydrazidato, phen = 1,10 phenanthroline, and bipy = 2,2′ bipyridine, were prepared and fully characterized using elemental analyses, FT-IR, molar conductivity, and electronic spectra. The structures of both complexes were also determined by X-ray diffraction. It was found that, both complexes possessed square pyramidal coordination environment in which, Cu(II) ions were coordinated by donor atoms of HL and two nitrogens of heterocyclic bases. Computational studies were performed using DFT calculations at B3LYP/6-311+G(d,p) level of theory. DNA binding activities of these complexes were also investigated using electronic absorption, competitive fluorescence titration and cyclic voltammetry studies. The obtained results indicated that binding of the complexes to DNA was of intercalative mode. Furthermore, antimicrobial activities of these compounds were screened against microorganisms.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide