| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229219 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 6 Pages |
•HB lengths and energies of C151-(H2O)5 for A, B and C type HBs at S0 and S1 states.•Additional HB between O of the pyrone ring and H of a water molecule.•One HB formed between two water molecules.•The A type HB energy decreased by 4.37 kJ/mol.•The B and C type HB energies are increased by 5.62 and 10.21 kJ/mol, respectively.
Change in energy of hydrogen bonds (HBs) upon excitation, plays an important role on the spectra of chemical and biological molecules. Effective fragment potential (EFP) method of explicit water molecules embedded in polarizable continuum medium (PCM) is used for the solvation of 7-Amino-4-(trifluoromethyl)coumarin (C151). Time dependent density functional theory (TDDFT) calculations combined with EFP/PCM had been carried out to study the electronic structure and the exited state properties of C151 with five water molecules (C151–(H2O)5 complex). S0 state and S1 state geometries were optimized using DFT/TDDFT with PBE0 functional combined with cc-pVDZ basis set, the transition energies are computed with same basis set and functional. Change in HB energy is calculated using the procedure proposed by T. Nagata et al. to calculate solute–solvent interaction energy in Nagata et al. (2011). Upon photoexcitation of C151–(H2O)5 complex, A type (N⋯HO) HB is weakened with decrease of energy by 4.37 kJ/mol, whereas B and C type (CO⋯HO and NH⋯O) HBs are strengthened with increase of 5.62 and 10.21 kJ/mol energy, respectively. This study again confirmed that the intermolecular hydrogen bonds between C151 chromophore and aqueous solvents are strengthened, not cleaved upon electronic excitation.
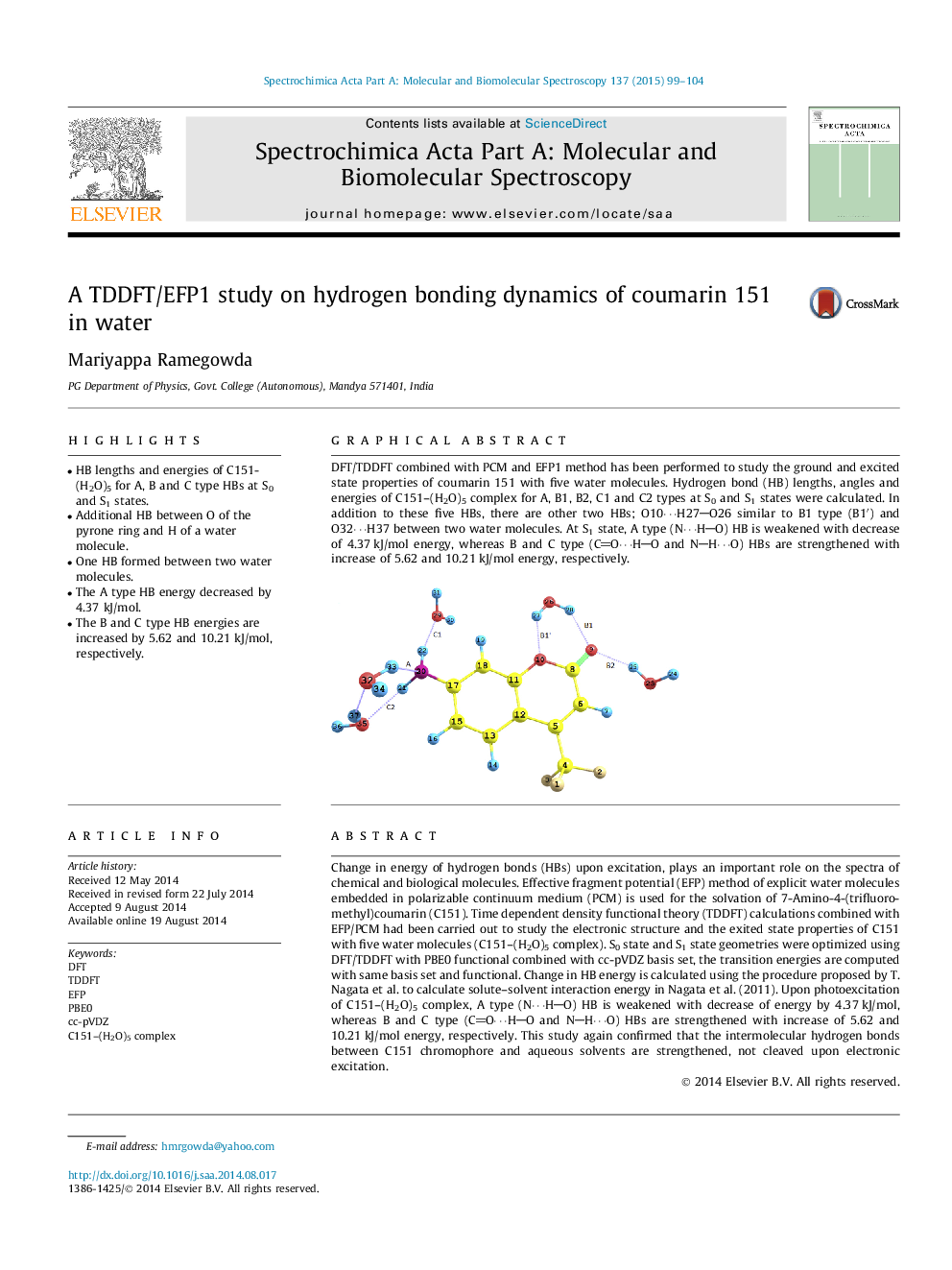
Graphical abstractDFT/TDDFT combined with PCM and EFP1 method has been performed to study the ground and excited state properties of coumarin 151 with five water molecules. Hydrogen bond (HB) lengths, angles and energies of C151–(H2O)5 complex for A, B1, B2, C1 and C2 types at S0 and S1 states were calculated. In addition to these five HBs, there are other two HBs; O10⋯H27O26 similar to B1 type (B1′) and O32⋯H37 between two water molecules. At S1 state, A type (N⋯HO) HB is weakened with decrease of 4.37 kJ/mol energy, whereas B and C type (CO⋯HO and NH⋯O) HBs are strengthened with increase of 5.62 and 10.21 kJ/mol energy, respectively.Figure optionsDownload full-size imageDownload as PowerPoint slide