| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229226 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 6 Pages |
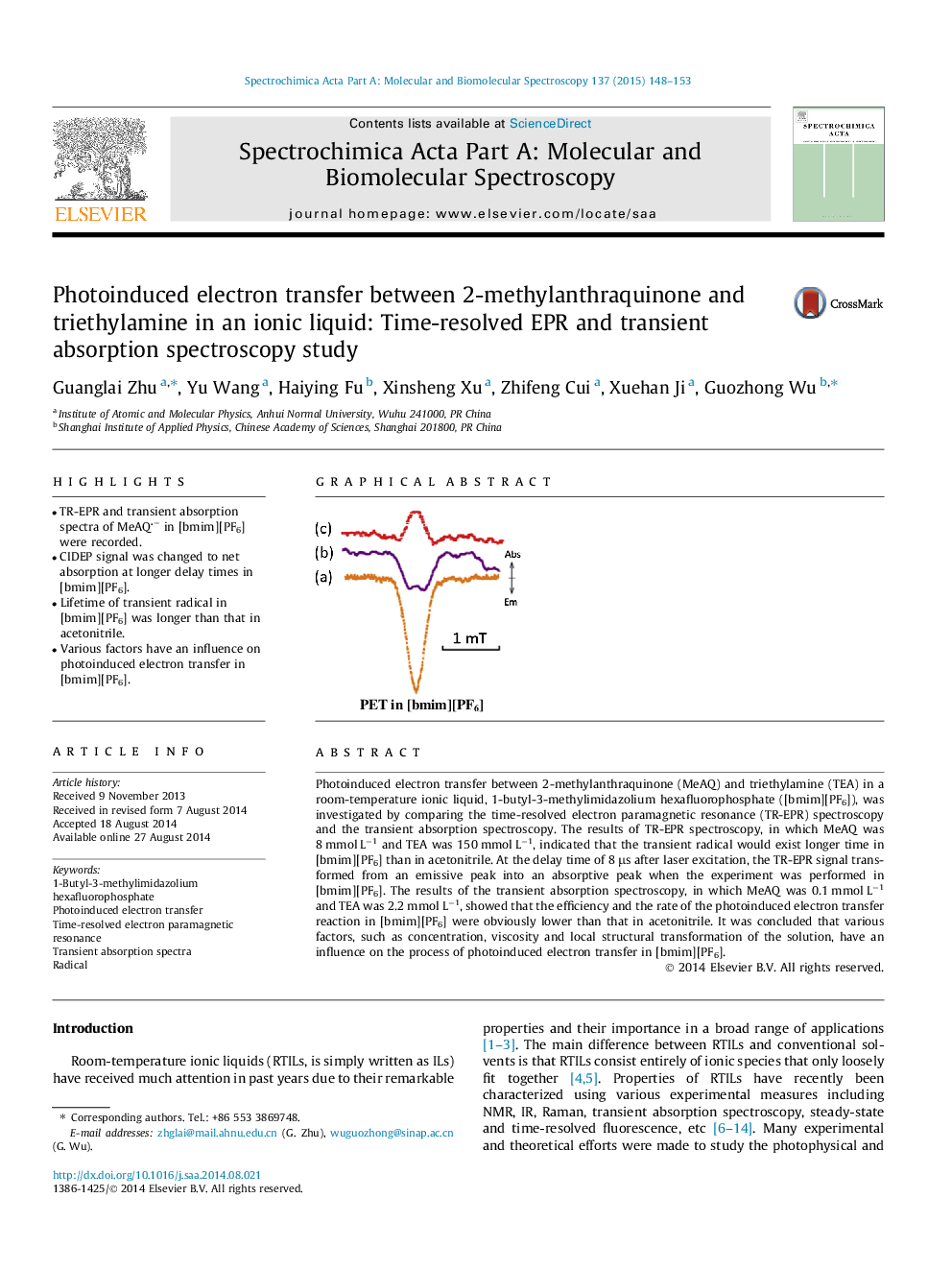
•TR-EPR and transient absorption spectra of MeAQ− in [bmim][PF6] were recorded.•CIDEP signal was changed to net absorption at longer delay times in [bmim][PF6].•Lifetime of transient radical in [bmim][PF6] was longer than that in acetonitrile.•Various factors have an influence on photoinduced electron transfer in [bmim][PF6].
Photoinduced electron transfer between 2-methylanthraquinone (MeAQ) and triethylamine (TEA) in a room-temperature ionic liquid, 1-butyl-3-methylimidazolium hexafluorophosphate ([bmim][PF6]), was investigated by comparing the time-resolved electron paramagnetic resonance (TR-EPR) spectroscopy and the transient absorption spectroscopy. The results of TR-EPR spectroscopy, in which MeAQ was 8 mmol L−1 and TEA was 150 mmol L−1, indicated that the transient radical would exist longer time in [bmim][PF6] than in acetonitrile. At the delay time of 8 μs after laser excitation, the TR-EPR signal transformed from an emissive peak into an absorptive peak when the experiment was performed in [bmim][PF6]. The results of the transient absorption spectroscopy, in which MeAQ was 0.1 mmol L−1 and TEA was 2.2 mmol L−1, showed that the efficiency and the rate of the photoinduced electron transfer reaction in [bmim][PF6] were obviously lower than that in acetonitrile. It was concluded that various factors, such as concentration, viscosity and local structural transformation of the solution, have an influence on the process of photoinduced electron transfer in [bmim][PF6].
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide