| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229264 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 8 Pages |
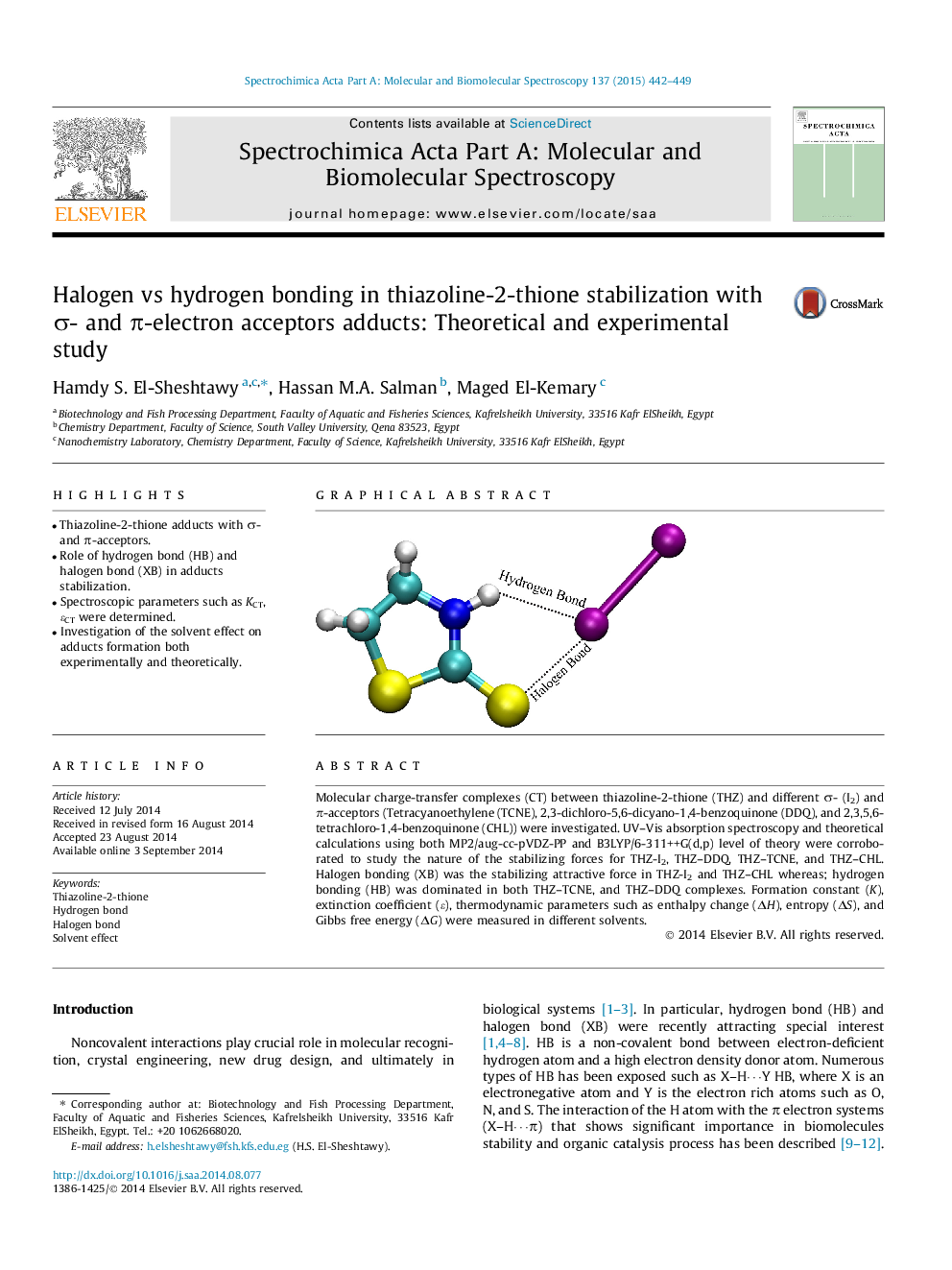
•Thiazoline-2-thione adducts with σ- and π-acceptors.•Role of hydrogen bond (HB) and halogen bond (XB) in adducts stabilization.•Spectroscopic parameters such as KCT, ɛCT were determined.•Investigation of the solvent effect on adducts formation both experimentally and theoretically.
Molecular charge-transfer complexes (CT) between thiazoline-2-thione (THZ) and different σ- (I2) and π-acceptors (Tetracyanoethylene (TCNE), 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ), and 2,3,5,6-tetrachloro-1,4-benzoquinone (CHL)) were investigated. UV–Vis absorption spectroscopy and theoretical calculations using both MP2/aug-cc-pVDZ-PP and B3LYP/6-311++G(d,p) level of theory were corroborated to study the nature of the stabilizing forces for THZ-I2, THZ–DDQ, THZ–TCNE, and THZ–CHL. Halogen bonding (XB) was the stabilizing attractive force in THZ-I2 and THZ–CHL whereas; hydrogen bonding (HB) was dominated in both THZ–TCNE, and THZ–DDQ complexes. Formation constant (K), extinction coefficient (ɛ), thermodynamic parameters such as enthalpy change (ΔH), entropy (ΔS), and Gibbs free energy (ΔG) were measured in different solvents.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide