| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229288 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 9 Pages |
•Conformers were found from theoretical calculations of diacetylenic acids.•FT-IR spectra measured by ATR technique and in transmission (KBr) were compared.•UV polymerization under various conditions was followed by Raman spectroscopy.•Fermi Resonance (FR) in IR spectra was suggested.•Calculations of dimers were applied for the interpretation of experimental spectra.
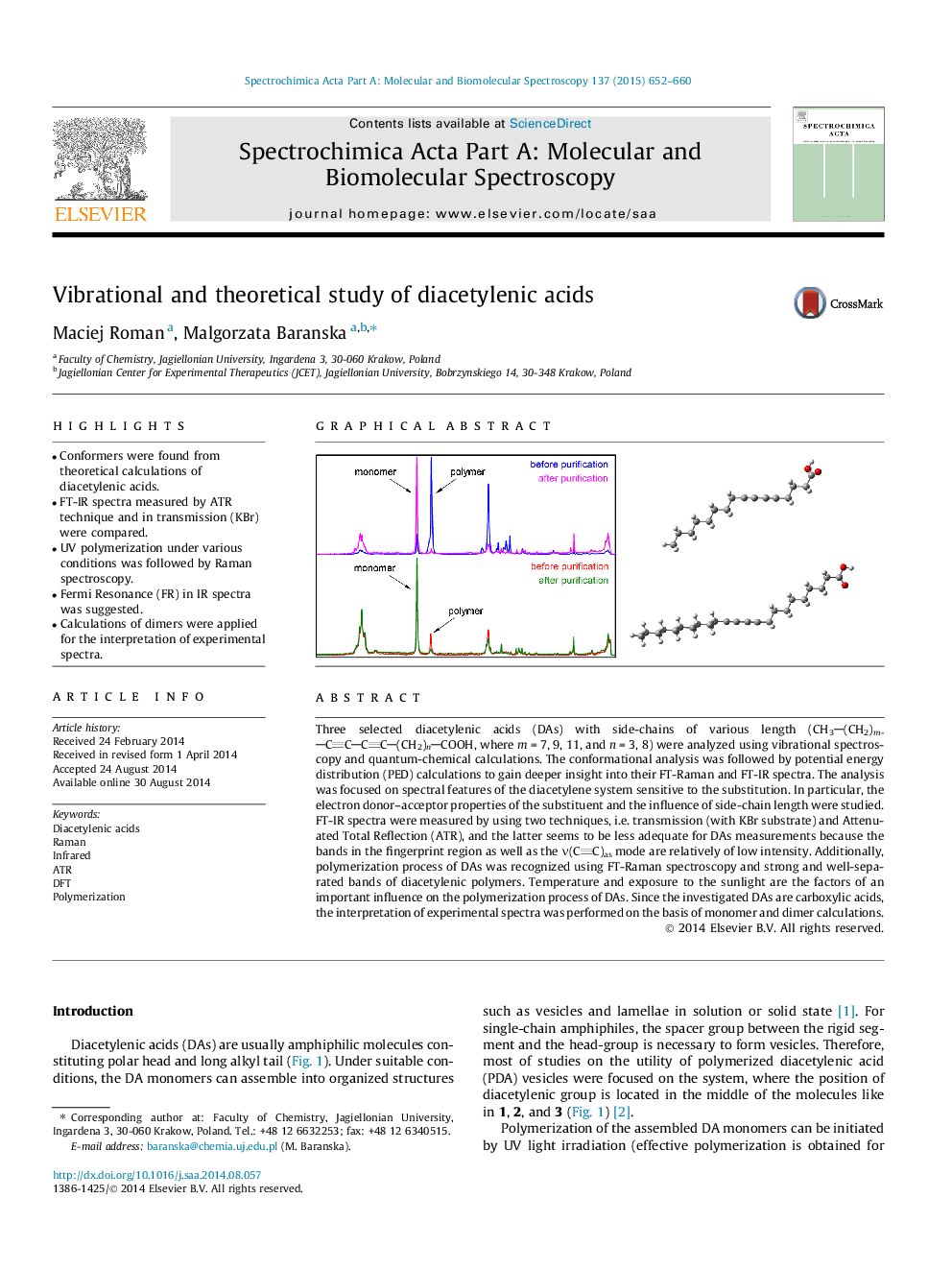
Three selected diacetylenic acids (DAs) with side-chains of various length (CH3(CH2)mCCCC(CH2)nCOOH, where m = 7, 9, 11, and n = 3, 8) were analyzed using vibrational spectroscopy and quantum-chemical calculations. The conformational analysis was followed by potential energy distribution (PED) calculations to gain deeper insight into their FT-Raman and FT-IR spectra. The analysis was focused on spectral features of the diacetylene system sensitive to the substitution. In particular, the electron donor–acceptor properties of the substituent and the influence of side-chain length were studied. FT-IR spectra were measured by using two techniques, i.e. transmission (with KBr substrate) and Attenuated Total Reflection (ATR), and the latter seems to be less adequate for DAs measurements because the bands in the fingerprint region as well as the ν(CC)as mode are relatively of low intensity. Additionally, polymerization process of DAs was recognized using FT-Raman spectroscopy and strong and well-separated bands of diacetylenic polymers. Temperature and exposure to the sunlight are the factors of an important influence on the polymerization process of DAs. Since the investigated DAs are carboxylic acids, the interpretation of experimental spectra was performed on the basis of monomer and dimer calculations.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide