| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229353 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 9 Pages |
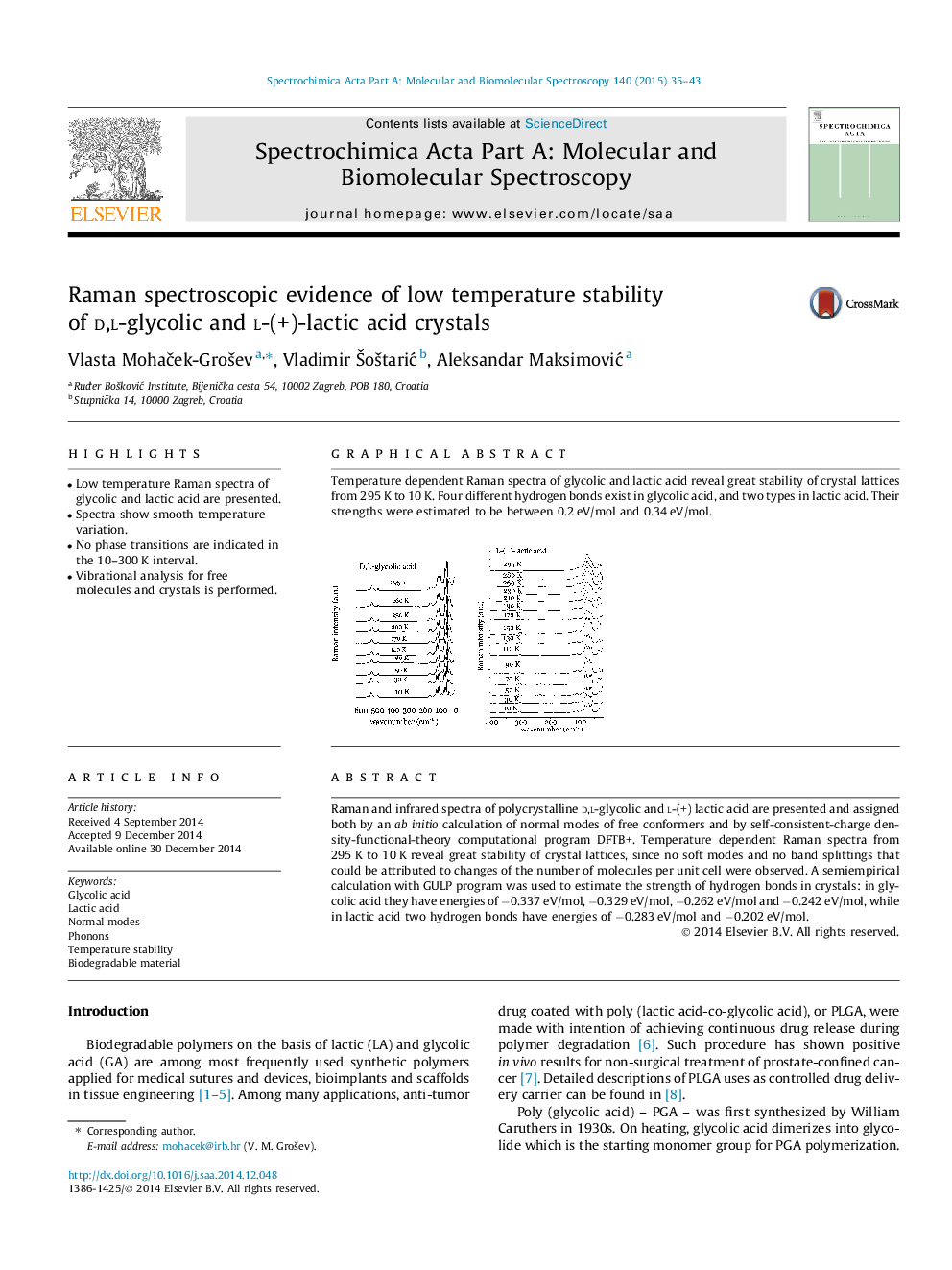
•Low temperature Raman spectra of glycolic and lactic acid are presented.•Spectra show smooth temperature variation.•No phase transitions are indicated in the 10–300 K interval.•Vibrational analysis for free molecules and crystals is performed.
Raman and infrared spectra of polycrystalline d,l-glycolic and l-(+) lactic acid are presented and assigned both by an ab initio calculation of normal modes of free conformers and by self-consistent-charge density-functional-theory computational program DFTB+. Temperature dependent Raman spectra from 295 K to 10 K reveal great stability of crystal lattices, since no soft modes and no band splittings that could be attributed to changes of the number of molecules per unit cell were observed. A semiempirical calculation with GULP program was used to estimate the strength of hydrogen bonds in crystals: in glycolic acid they have energies of −0.337 eV/mol, −0.329 eV/mol, −0.262 eV/mol and −0.242 eV/mol, while in lactic acid two hydrogen bonds have energies of −0.283 eV/mol and −0.202 eV/mol.
Graphical abstractTemperature dependent Raman spectra of glycolic and lactic acid reveal great stability of crystal lattices from 295 K to 10 K. Four different hydrogen bonds exist in glycolic acid, and two types in lactic acid. Their strengths were estimated to be between 0.2 eV/mol and 0.34 eV/mol.Figure optionsDownload full-size imageDownload as PowerPoint slide