| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229359 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 5 Pages |
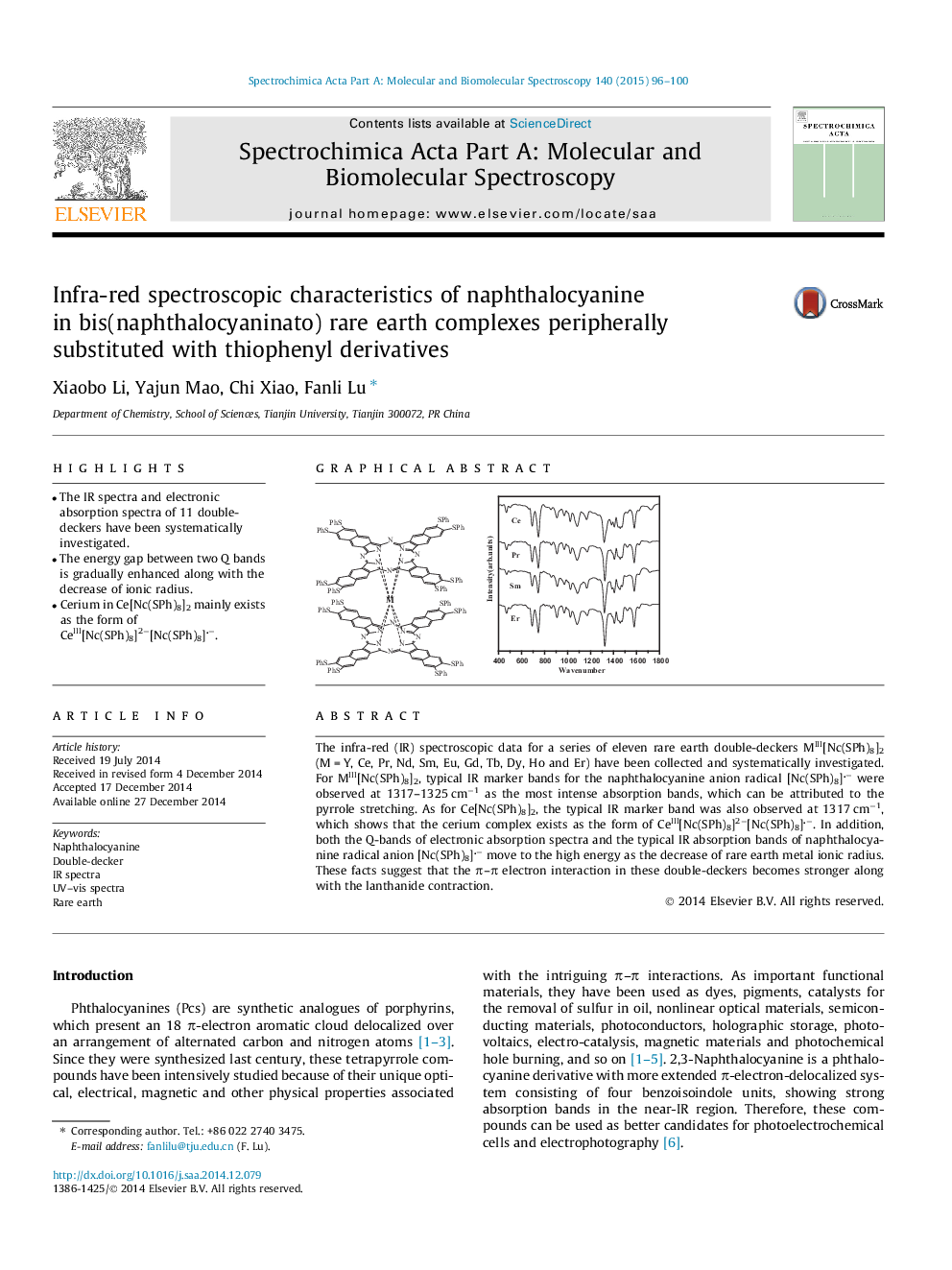
•The IR spectra and electronic absorption spectra of 11 double-deckers have been systematically investigated.•The energy gap between two Q bands is gradually enhanced along with the decrease of ionic radius.•Cerium in Ce[Nc(SPh)8]2 mainly exists as the form of CeIII[Nc(SPh)8]2−[Nc(SPh)8]−.
The infra-red (IR) spectroscopic data for a series of eleven rare earth double-deckers MIII[Nc(SPh)8]2 (M = Y, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho and Er) have been collected and systematically investigated. For MIII[Nc(SPh)8]2, typical IR marker bands for the naphthalocyanine anion radical [Nc(SPh)8]− were observed at 1317–1325 cm−1 as the most intense absorption bands, which can be attributed to the pyrrole stretching. As for Ce[Nc(SPh)8]2, the typical IR marker band was also observed at 1317 cm−1, which shows that the cerium complex exists as the form of CeIII[Nc(SPh)8]2−[Nc(SPh)8]−. In addition, both the Q-bands of electronic absorption spectra and the typical IR absorption bands of naphthalocyanine radical anion [Nc(SPh)8]− move to the high energy as the decrease of rare earth metal ionic radius. These facts suggest that the π–π electron interaction in these double-deckers becomes stronger along with the lanthanide contraction.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide