| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229453 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 11 Pages |
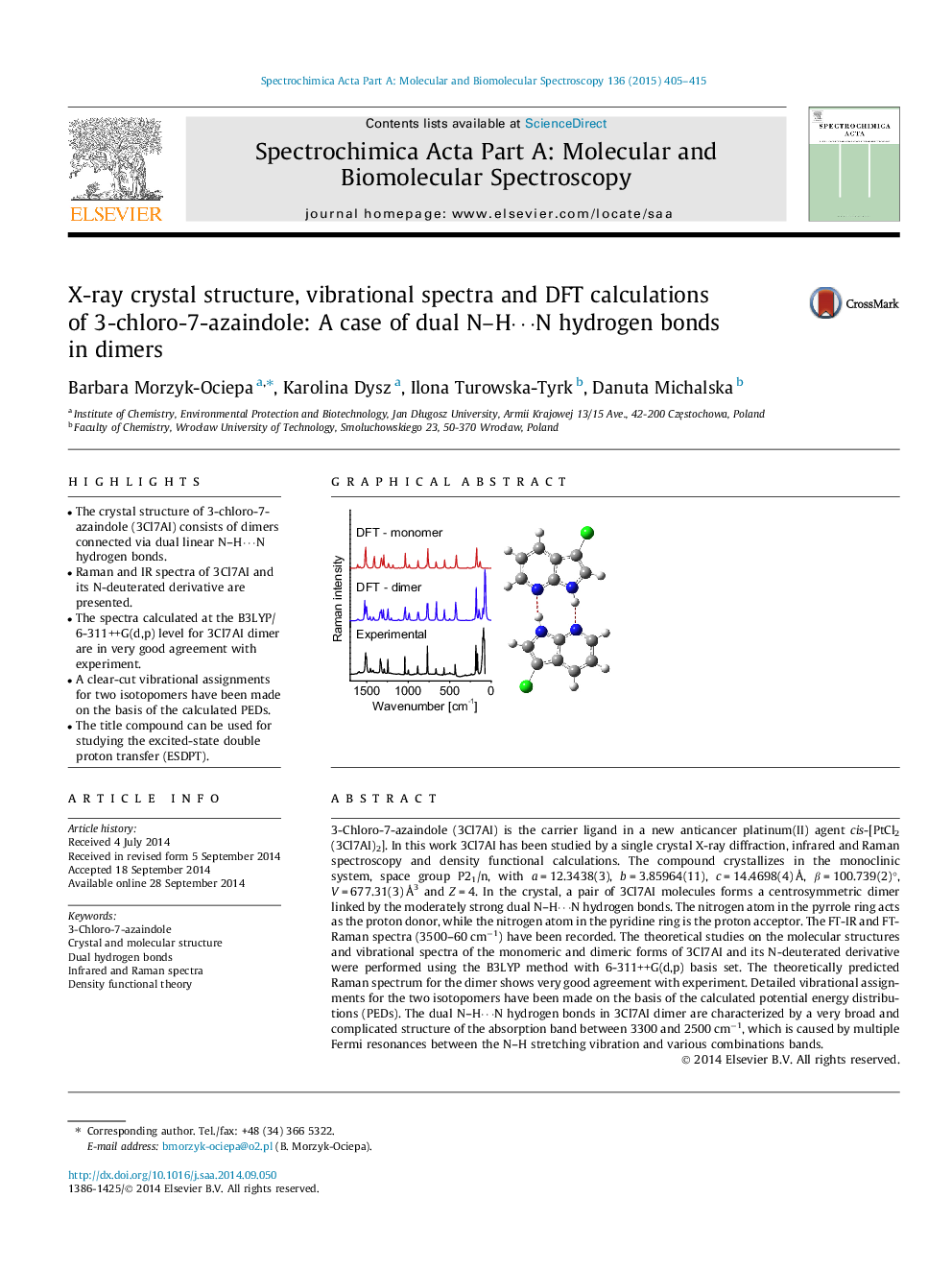
•The crystal structure of 3-chloro-7-azaindole (3Cl7AI) consists of dimers connected via dual linear N–H⋯N hydrogen bonds.•Raman and IR spectra of 3Cl7AI and its N-deuterated derivative are presented.•The spectra calculated at the B3LYP/6-311++G(d,p) level for 3Cl7AI dimer are in very good agreement with experiment.•A clear-cut vibrational assignments for two isotopomers have been made on the basis of the calculated PEDs.•The title compound can be used for studying the excited-state double proton transfer (ESDPT).
3-Chloro-7-azaindole (3Cl7AI) is the carrier ligand in a new anticancer platinum(II) agent cis-[PtCl2(3Cl7AI)2]. In this work 3Cl7AI has been studied by a single crystal X-ray diffraction, infrared and Raman spectroscopy and density functional calculations. The compound crystallizes in the monoclinic system, space group P21/n, with a = 12.3438(3), b = 3.85964(11), c = 14.4698(4) Å, β = 100.739(2)°, V = 677.31(3) Å3 and Z = 4. In the crystal, a pair of 3Cl7AI molecules forms a centrosymmetric dimer linked by the moderately strong dual N–H⋯N hydrogen bonds. The nitrogen atom in the pyrrole ring acts as the proton donor, while the nitrogen atom in the pyridine ring is the proton acceptor. The FT-IR and FT-Raman spectra (3500–60 cm−1) have been recorded. The theoretical studies on the molecular structures and vibrational spectra of the monomeric and dimeric forms of 3Cl7AI and its N-deuterated derivative were performed using the B3LYP method with 6-311++G(d,p) basis set. The theoretically predicted Raman spectrum for the dimer shows very good agreement with experiment. Detailed vibrational assignments for the two isotopomers have been made on the basis of the calculated potential energy distributions (PEDs). The dual N–H⋯N hydrogen bonds in 3Cl7AI dimer are characterized by a very broad and complicated structure of the absorption band between 3300 and 2500 cm−1, which is caused by multiple Fermi resonances between the N–H stretching vibration and various combinations bands.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide