| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229476 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 10 Pages |
•Kinetic of thermal decomposition showed that the complexes had good thermal stability.•Electrochemical studies showed the difference in redox potential of the complexes according to the substitutional groups.•The X-ray of vanadyl Schiff base complex showed two different crystal structure.
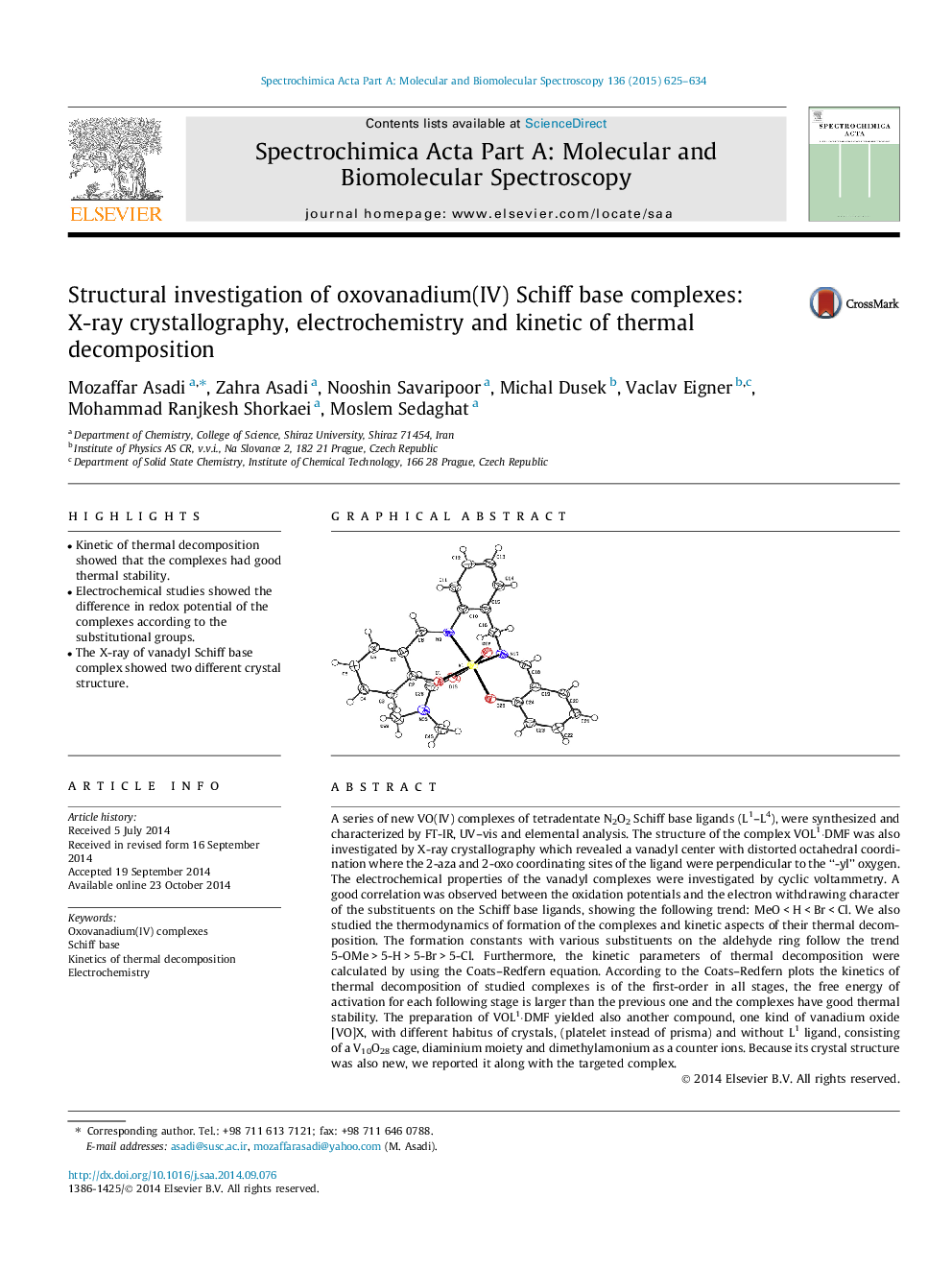
A series of new VO(IV) complexes of tetradentate N2O2 Schiff base ligands (L1–L4), were synthesized and characterized by FT-IR, UV–vis and elemental analysis. The structure of the complex VOL1⋅DMF was also investigated by X-ray crystallography which revealed a vanadyl center with distorted octahedral coordination where the 2-aza and 2-oxo coordinating sites of the ligand were perpendicular to the “-yl” oxygen. The electrochemical properties of the vanadyl complexes were investigated by cyclic voltammetry. A good correlation was observed between the oxidation potentials and the electron withdrawing character of the substituents on the Schiff base ligands, showing the following trend: MeO < H < Br < Cl. We also studied the thermodynamics of formation of the complexes and kinetic aspects of their thermal decomposition. The formation constants with various substituents on the aldehyde ring follow the trend 5-OMe > 5-H > 5-Br > 5-Cl. Furthermore, the kinetic parameters of thermal decomposition were calculated by using the Coats–Redfern equation. According to the Coats–Redfern plots the kinetics of thermal decomposition of studied complexes is of the first-order in all stages, the free energy of activation for each following stage is larger than the previous one and the complexes have good thermal stability. The preparation of VOL1⋅DMF yielded also another compound, one kind of vanadium oxide [VO]X, with different habitus of crystals, (platelet instead of prisma) and without L1 ligand, consisting of a V10O28 cage, diaminium moiety and dimethylamonium as a counter ions. Because its crystal structure was also new, we reported it along with the targeted complex.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide