| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229478 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 7 Pages |
•1, 2, 3 and 4 molecules were optimized by using DFT method.•The effects of halogen atoms on electric properties were investigated.•It was obtained to be β∝1η.•The possibility of charge transfer is increased by the reduction in η.
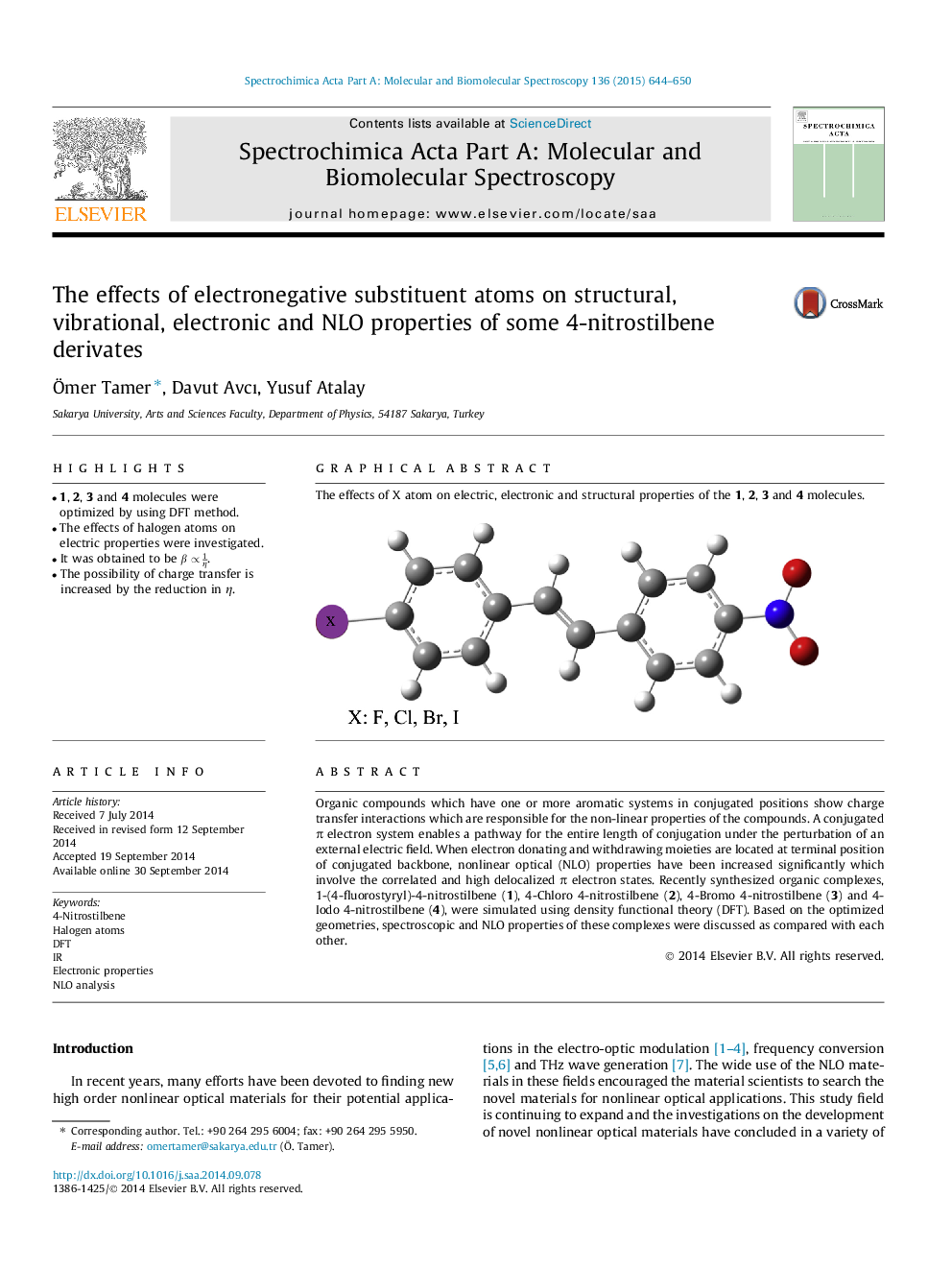
Organic compounds which have one or more aromatic systems in conjugated positions show charge transfer interactions which are responsible for the non-linear properties of the compounds. A conjugated π electron system enables a pathway for the entire length of conjugation under the perturbation of an external electric field. When electron donating and withdrawing moieties are located at terminal position of conjugated backbone, nonlinear optical (NLO) properties have been increased significantly which involve the correlated and high delocalized π electron states. Recently synthesized organic complexes, 1-(4-fluorostyryl)-4-nitrostilbene (1), 4-Chloro 4-nitrostilbene (2), 4-Bromo 4-nitrostilbene (3) and 4-Iodo 4-nitrostilbene (4), were simulated using density functional theory (DFT). Based on the optimized geometries, spectroscopic and NLO properties of these complexes were discussed as compared with each other.
Graphical abstractThe effects of X atom on electric, electronic and structural properties of the 1, 2, 3 and 4 molecules.Figure optionsDownload full-size imageDownload as PowerPoint slide