| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229498 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 6 Pages |
•A new N-Methyl benzimidazolyl Schiff base ligand has been synthesized & characterized structurally.•Copper(II) complexes of this ligand have been synthesized and characterized spectroscopically.•The complexes are utilized heterogeneously for the oxidation of 1-phenyl propyne.•SEM and PXRD are used to observe changes in catalytic activity upon reuse of catalyst.
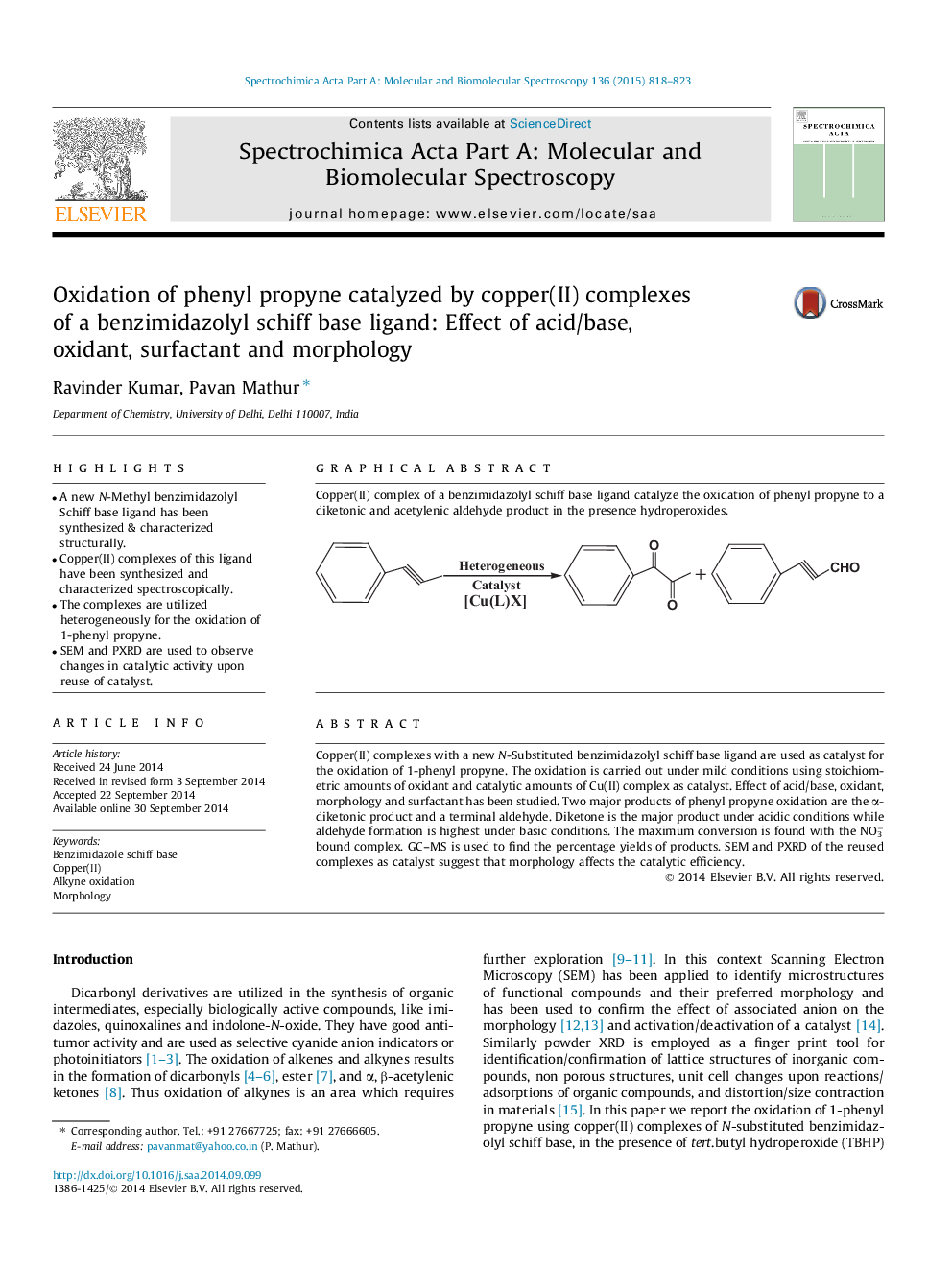
Copper(II) complexes with a new N-Substituted benzimidazolyl schiff base ligand are used as catalyst for the oxidation of 1-phenyl propyne. The oxidation is carried out under mild conditions using stoichiometric amounts of oxidant and catalytic amounts of Cu(II) complex as catalyst. Effect of acid/base, oxidant, morphology and surfactant has been studied. Two major products of phenyl propyne oxidation are the α-diketonic product and a terminal aldehyde. Diketone is the major product under acidic conditions while aldehyde formation is highest under basic conditions. The maximum conversion is found with the NO3− bound complex. GC–MS is used to find the percentage yields of products. SEM and PXRD of the reused complexes as catalyst suggest that morphology affects the catalytic efficiency.
Graphical abstractCopper(II) complex of a benzimidazolyl schiff base ligand catalyze the oxidation of phenyl propyne to a diketonic and acetylenic aldehyde product in the presence hydroperoxides.Figure optionsDownload full-size imageDownload as PowerPoint slide