| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229515 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 16 Pages |
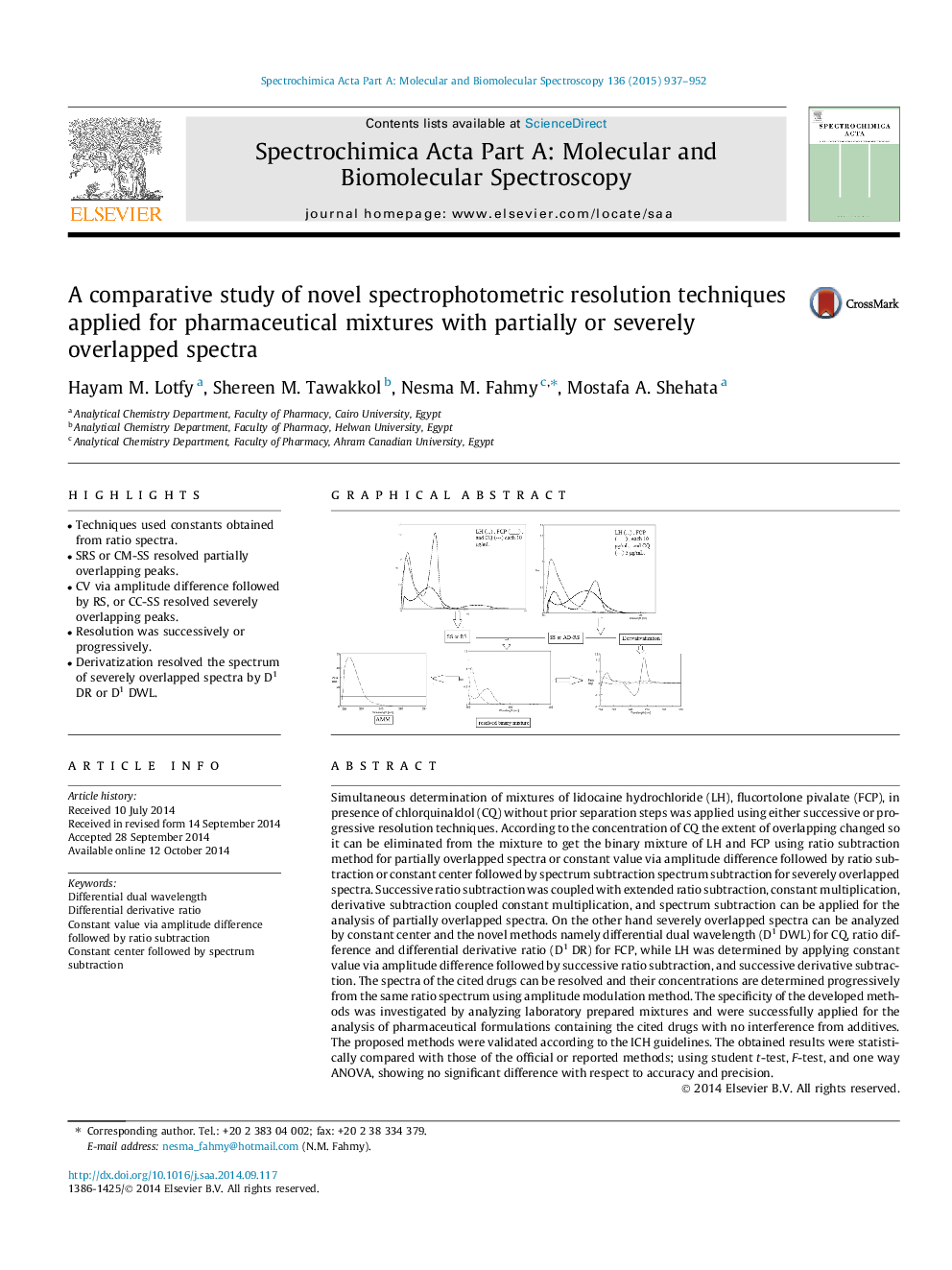
•Techniques used constants obtained from ratio spectra.•SRS or CM-SS resolved partially overlapping peaks.•CV via amplitude difference followed by RS, or CC-SS resolved severely overlapping peaks.•Resolution was successively or progressively.•Derivatization resolved the spectrum of severely overlapped spectra by D1 DR or D1 DWL.
Simultaneous determination of mixtures of lidocaine hydrochloride (LH), flucortolone pivalate (FCP), in presence of chlorquinaldol (CQ) without prior separation steps was applied using either successive or progressive resolution techniques. According to the concentration of CQ the extent of overlapping changed so it can be eliminated from the mixture to get the binary mixture of LH and FCP using ratio subtraction method for partially overlapped spectra or constant value via amplitude difference followed by ratio subtraction or constant center followed by spectrum subtraction spectrum subtraction for severely overlapped spectra. Successive ratio subtraction was coupled with extended ratio subtraction, constant multiplication, derivative subtraction coupled constant multiplication, and spectrum subtraction can be applied for the analysis of partially overlapped spectra. On the other hand severely overlapped spectra can be analyzed by constant center and the novel methods namely differential dual wavelength (D1 DWL) for CQ, ratio difference and differential derivative ratio (D1 DR) for FCP, while LH was determined by applying constant value via amplitude difference followed by successive ratio subtraction, and successive derivative subtraction. The spectra of the cited drugs can be resolved and their concentrations are determined progressively from the same ratio spectrum using amplitude modulation method. The specificity of the developed methods was investigated by analyzing laboratory prepared mixtures and were successfully applied for the analysis of pharmaceutical formulations containing the cited drugs with no interference from additives. The proposed methods were validated according to the ICH guidelines. The obtained results were statistically compared with those of the official or reported methods; using student t-test, F-test, and one way ANOVA, showing no significant difference with respect to accuracy and precision.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide