| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229555 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 5 Pages |
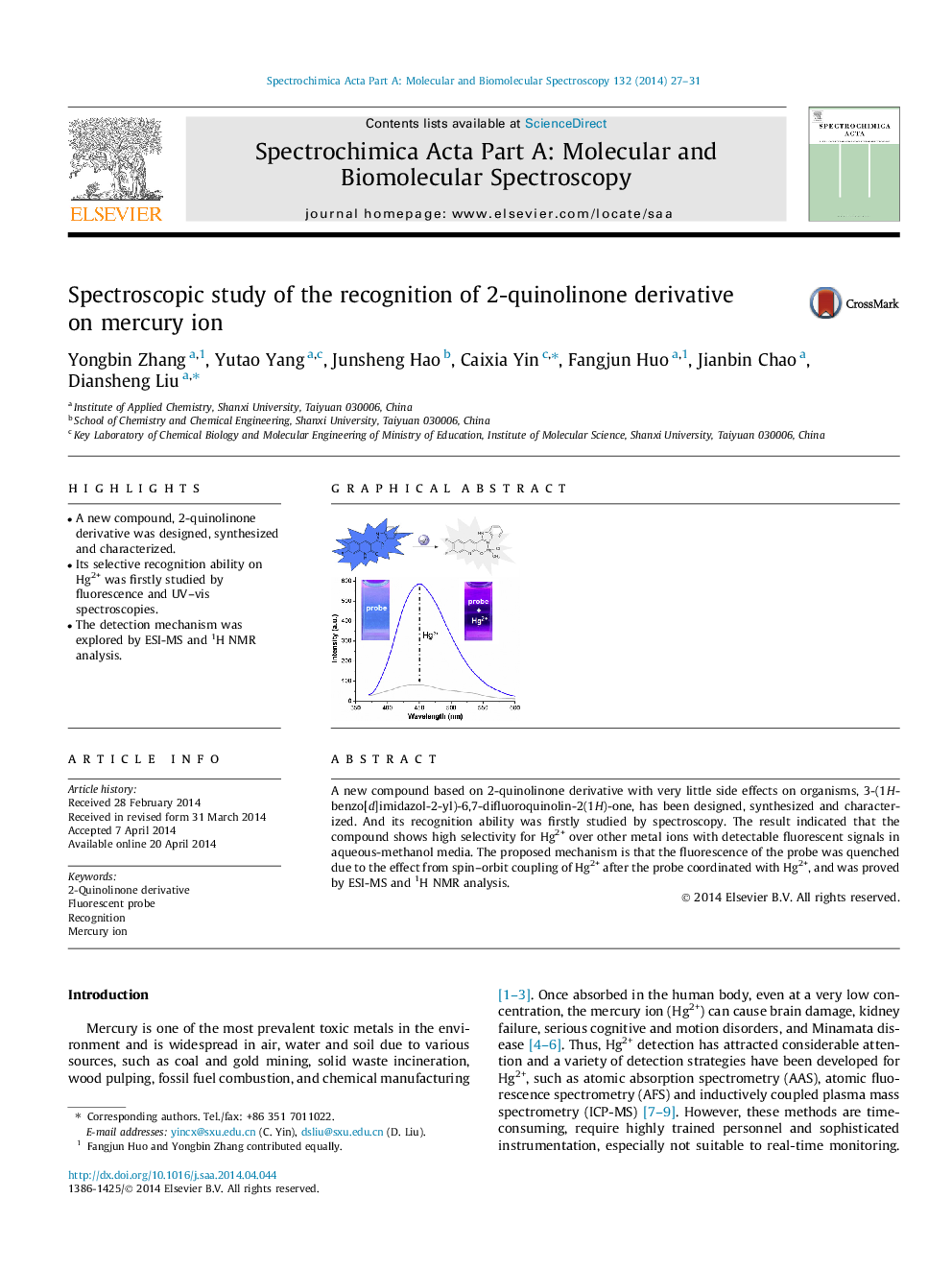
•A new compound, 2-quinolinone derivative was designed, synthesized and characterized.•Its selective recognition ability on Hg2+ was firstly studied by fluorescence and UV–vis spectroscopies.•The detection mechanism was explored by ESI-MS and 1H NMR analysis.
A new compound based on 2-quinolinone derivative with very little side effects on organisms, 3-(1H-benzo[d]imidazol-2-yl)-6,7-difluoroquinolin-2(1H)-one, has been designed, synthesized and characterized. And its recognition ability was firstly studied by spectroscopy. The result indicated that the compound shows high selectivity for Hg2+ over other metal ions with detectable fluorescent signals in aqueous-methanol media. The proposed mechanism is that the fluorescence of the probe was quenched due to the effect from spin–orbit coupling of Hg2+ after the probe coordinated with Hg2+, and was proved by ESI-MS and 1H NMR analysis.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide