| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229573 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 8 Pages |
•The compound exhibits strong effective intra- and intermolecular charge transfer.•The different electron-donor and -acceptor groups on the structural and electronic properties have been investigated.•NLO analysis shows that the title compound can be used as an effective NLO material.•The lowering of HOMO–LUMO energy band gap supports bioactive property of the molecule.•Small HOMO–LUMO energy gap implies that charge transfer occurs in the compound.
Quantum chemical calculations on the geometric parameters, harmonic vibrational wavenumbers and 1H and 13C nuclear magnetic resonance (NMR) chemical shifts values of 4-(methoxymethyl)-6-methyl-5-nitro-2-oxo-1,2-dihydropyridine-3-carbonitrile [C9H9N3O4] molecule in ground state were performed using the ab initio HF and density functional theory (DFT/B3LYP) methods with 6-311++G(d,p) basis set. The results of optimized molecular structure were presented and compared with X-ray diffraction results. The theoretical vibrational frequencies and 1H and 13C NMR chemical shifts values were compared with experimental values of the investigated molecule. The observed and calculated values were found to be in good agreement. Since the title compound contains different electron-donor and -acceptor groups as well as lone pair electrons, and multiple bonds, the effects of these groups on the structural and electronic properties are found out. In addition, conformational, natural bond orbital (NBO), nonlinear optical (NLO) analysis, frontier molecular orbital energies, molecular surfaces, Mulliken charges and atomic polar tensor based charges were investigated using HF and DFT methods.
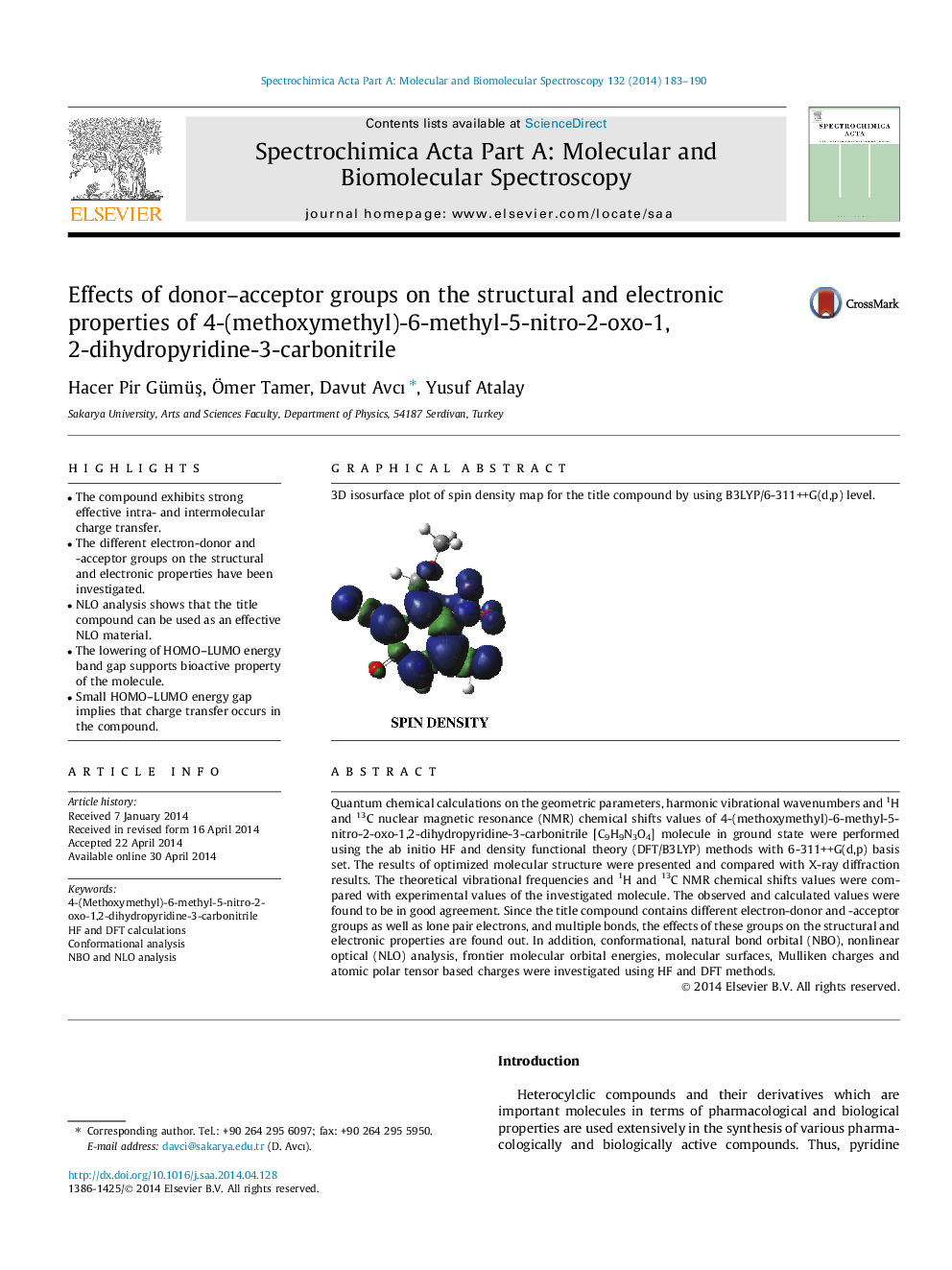
Graphical abstract3D isosurface plot of spin density map for the title compound by using B3LYP/6-311++G(d,p) level.Figure optionsDownload full-size imageDownload as PowerPoint slide