| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229585 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 10 Pages |
•1-Benzoyl-4-phenyl-3-thiosemicarbazide, N-phenyl-5-phenyl-1, 3, 4-thiadiazole-2-amine and 4, 5-diphenyl-2, 4-dihydro-1, 2, 4-triazole-3-thione have been synthesized.•The cyclization of thiosemicarbazide group in 1-benzoyl-4-phenyl-3- thiosemicarbazide are investigated by using FT-IR, Raman and DFT method.•The optimized geometries and MEPS plots performed by DFT method are reported.•HOMO and LUMO have negative energies indicating the stability of synthesized compounds.
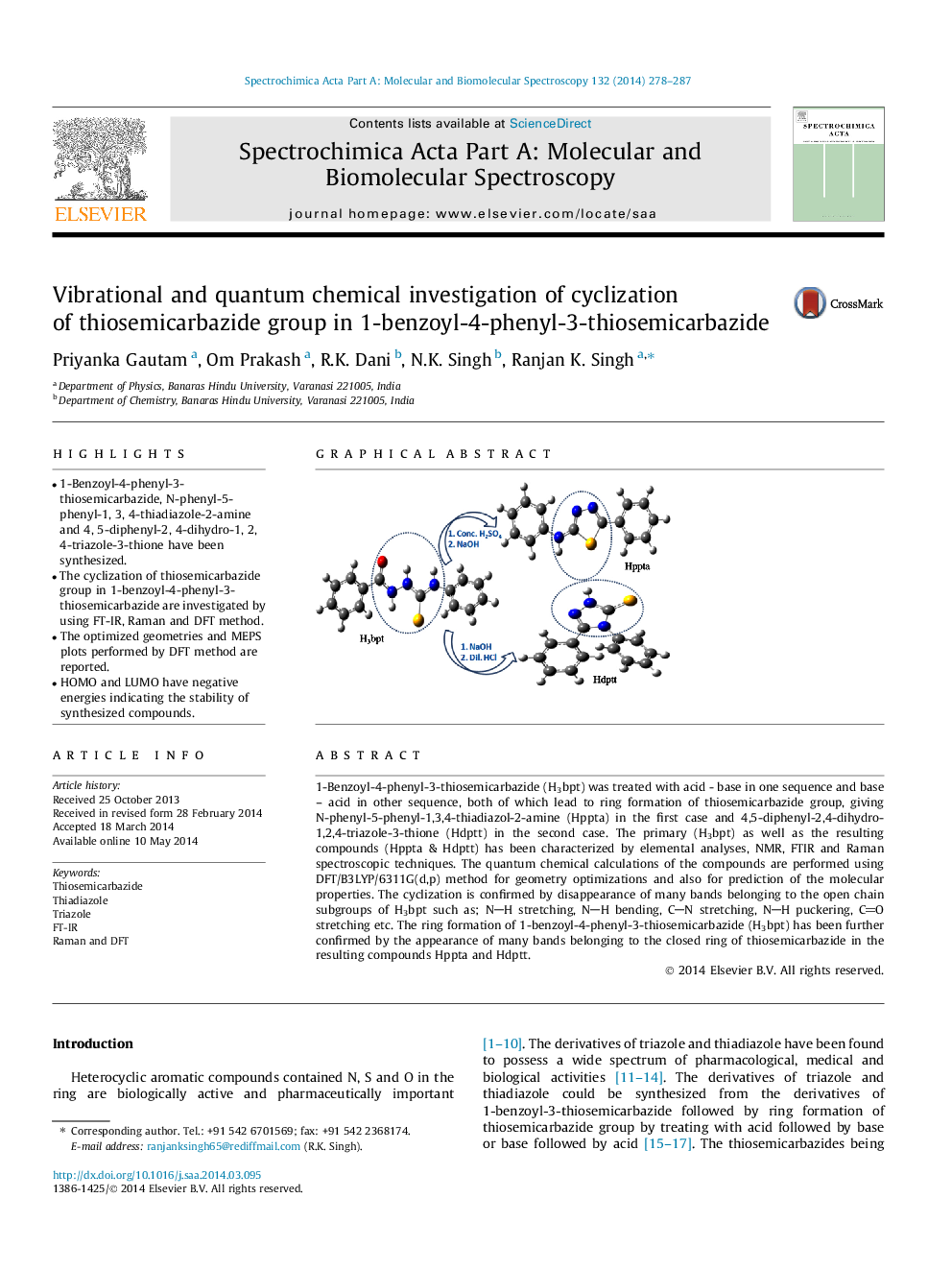
1-Benzoyl-4-phenyl-3-thiosemicarbazide (H3bpt) was treated with acid - base in one sequence and base – acid in other sequence, both of which lead to ring formation of thiosemicarbazide group, giving N-phenyl-5-phenyl-1,3,4-thiadiazol-2-amine (Hppta) in the first case and 4,5-diphenyl-2,4-dihydro-1,2,4-triazole-3-thione (Hdptt) in the second case. The primary (H3bpt) as well as the resulting compounds (Hppta & Hdptt) has been characterized by elemental analyses, NMR, FTIR and Raman spectroscopic techniques. The quantum chemical calculations of the compounds are performed using DFT/B3LYP/6311G(d,p) method for geometry optimizations and also for prediction of the molecular properties. The cyclization is confirmed by disappearance of many bands belonging to the open chain subgroups of H3bpt such as; NH stretching, NH bending, CN stretching, NH puckering, CO stretching etc. The ring formation of 1-benzoyl-4-phenyl-3-thiosemicarbazide (H3bpt) has been further confirmed by the appearance of many bands belonging to the closed ring of thiosemicarbazide in the resulting compounds Hppta and Hdptt.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide