| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229635 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 6 Pages |
•New symmetrical 1,2,5-oxadiazole based azo–azomethine dyes.•Effect of substituent, solvent, pH, temperature, H-bond, dipole moment on solvatochromic.•Increasing the antibacterial activity of azo–azomethine dyes with electron releasing of substituents.
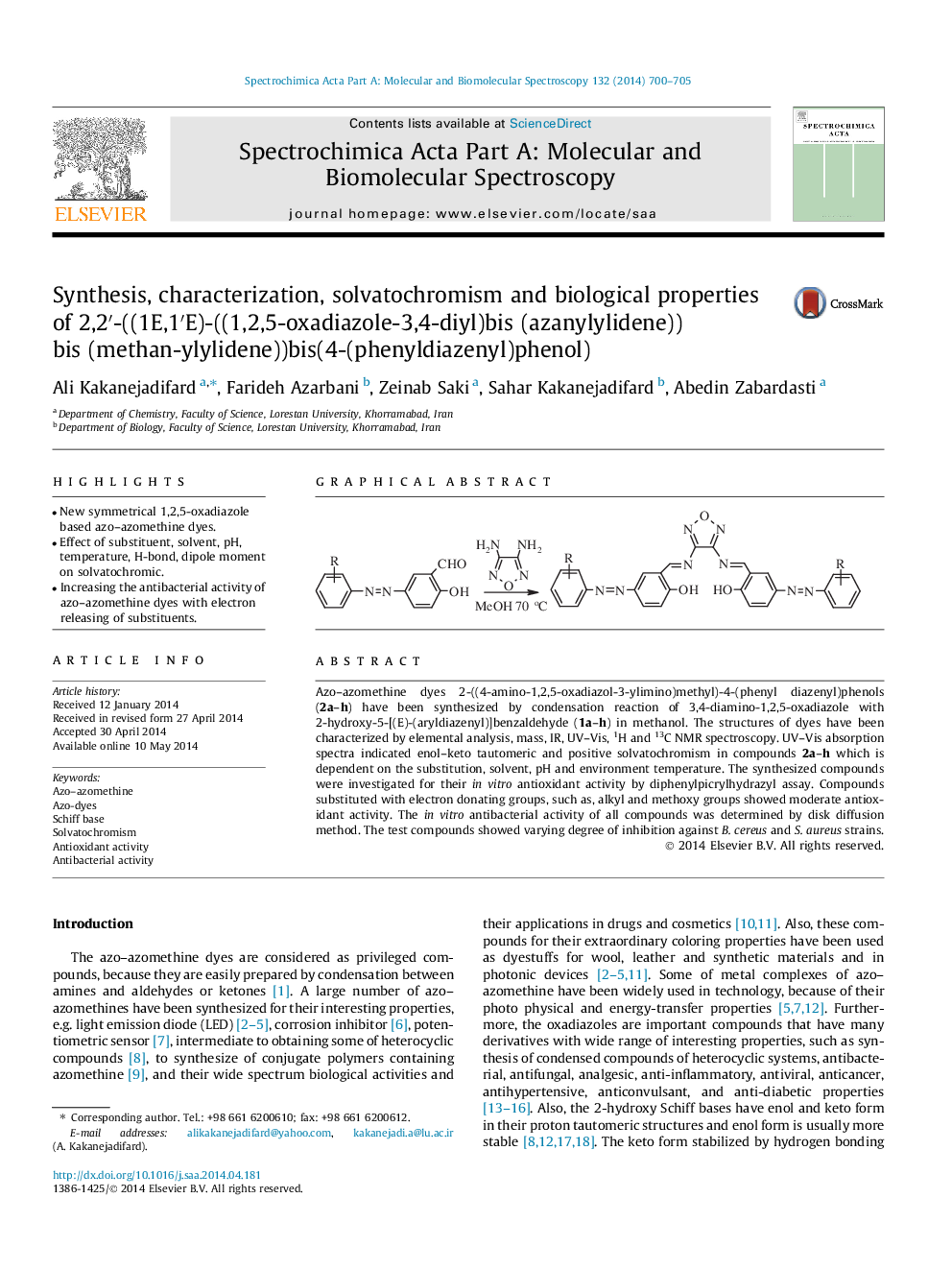
Azo–azomethine dyes 2-((4-amino-1,2,5-oxadiazol-3-ylimino)methyl)-4-(phenyl diazenyl)phenols (2a–h) have been synthesized by condensation reaction of 3,4-diamino-1,2,5-oxadiazole with 2-hydroxy-5-[(E)-(aryldiazenyl)]benzaldehyde (1a–h) in methanol. The structures of dyes have been characterized by elemental analysis, mass, IR, UV–Vis, 1H and 13С NMR spectroscopy. UV–Vis absorption spectra indicated enol–keto tautomeric and positive solvatochromism in compounds 2a–h which is dependent on the substitution, solvent, pH and environment temperature. The synthesized compounds were investigated for their in vitro antioxidant activity by diphenylpicrylhydrazyl assay. Compounds substituted with electron donating groups, such as, alkyl and methoxy groups showed moderate antioxidant activity. The in vitro antibacterial activity of all compounds was determined by disk diffusion method. The test compounds showed varying degree of inhibition against B. cereus and S. aureus strains.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide