| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229644 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 5 Pages |
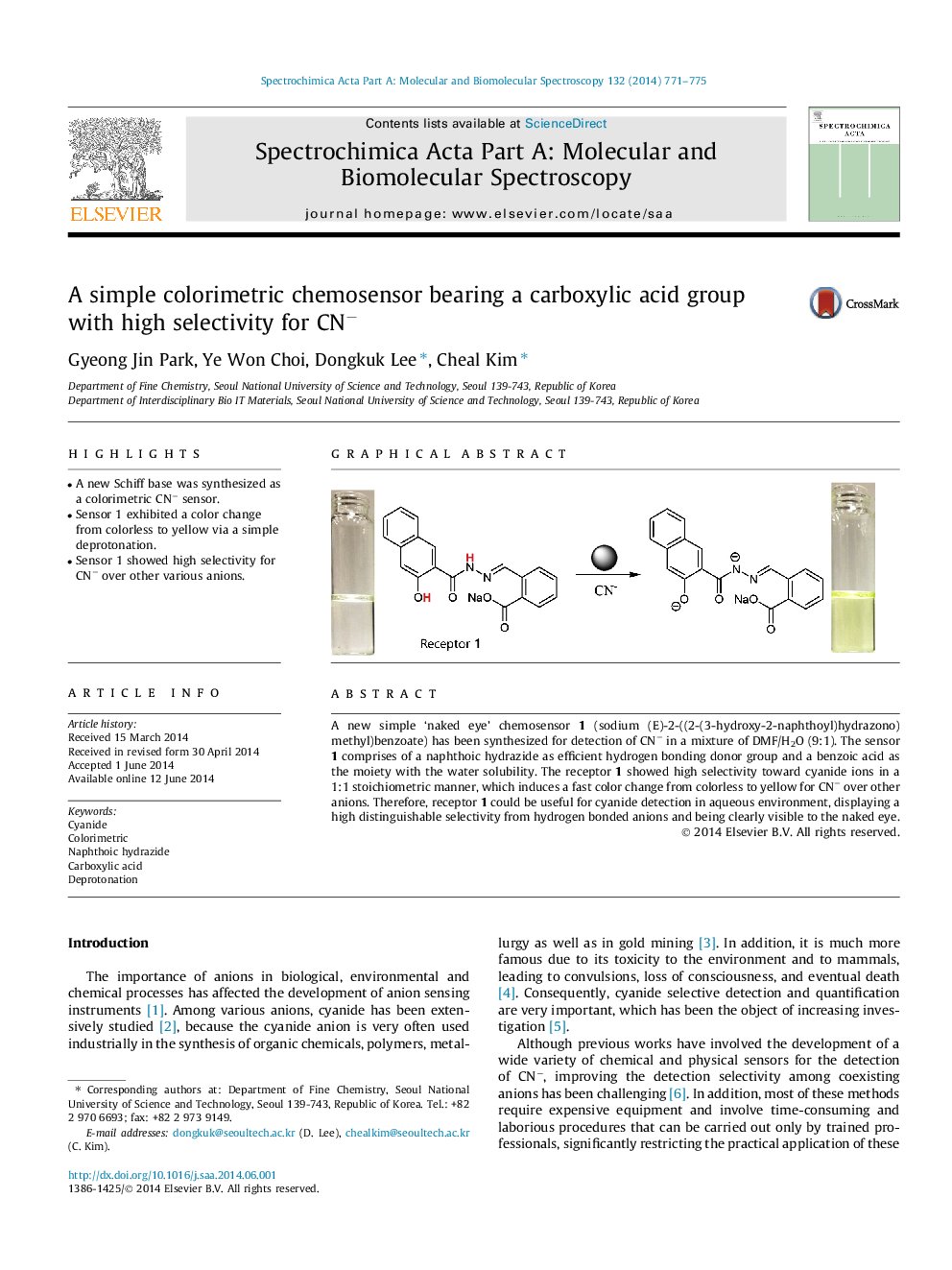
•A new Schiff base was synthesized as a colorimetric CN− sensor.•Sensor 1 exhibited a color change from colorless to yellow via a simple deprotonation.•Sensor 1 showed high selectivity for CN− over other various anions.
A new simple ‘naked eye’ chemosensor 1 (sodium (E)-2-((2-(3-hydroxy-2-naphthoyl)hydrazono)methyl)benzoate) has been synthesized for detection of CN− in a mixture of DMF/H2O (9:1). The sensor 1 comprises of a naphthoic hydrazide as efficient hydrogen bonding donor group and a benzoic acid as the moiety with the water solubility. The receptor 1 showed high selectivity toward cyanide ions in a 1:1 stoichiometric manner, which induces a fast color change from colorless to yellow for CN− over other anions. Therefore, receptor 1 could be useful for cyanide detection in aqueous environment, displaying a high distinguishable selectivity from hydrogen bonded anions and being clearly visible to the naked eye.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide