| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229649 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2014 | 6 Pages |
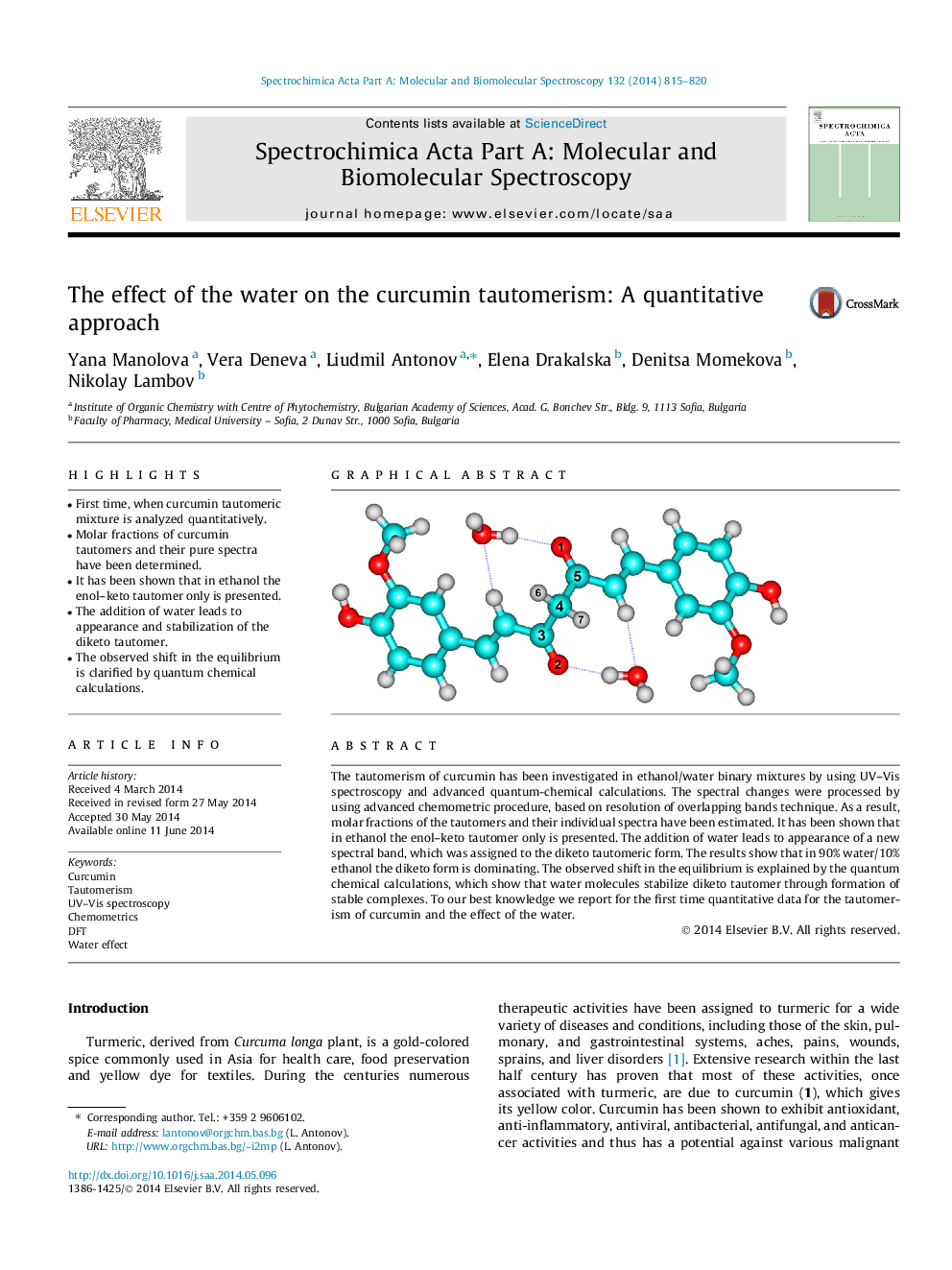
•First time, when curcumin tautomeric mixture is analyzed quantitatively.•Molar fractions of curcumin tautomers and their pure spectra have been determined.•It has been shown that in ethanol the enol–keto tautomer only is presented.•The addition of water leads to appearance and stabilization of the diketo tautomer.•The observed shift in the equilibrium is clarified by quantum chemical calculations.
The tautomerism of curcumin has been investigated in ethanol/water binary mixtures by using UV–Vis spectroscopy and advanced quantum-chemical calculations. The spectral changes were processed by using advanced chemometric procedure, based on resolution of overlapping bands technique. As a result, molar fractions of the tautomers and their individual spectra have been estimated. It has been shown that in ethanol the enol–keto tautomer only is presented. The addition of water leads to appearance of a new spectral band, which was assigned to the diketo tautomeric form. The results show that in 90% water/10% ethanol the diketo form is dominating. The observed shift in the equilibrium is explained by the quantum chemical calculations, which show that water molecules stabilize diketo tautomer through formation of stable complexes. To our best knowledge we report for the first time quantitative data for the tautomerism of curcumin and the effect of the water.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide