| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229658 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 6 Pages |
•1,2-Diphenyl-4-(3-methoxyphenyl)-1,3-cyclopentadiene was synthesized and characterized.•Aggregation-induced emission enhancement (AIEE) phenomena were investigated.•A combination effect of J-aggregation and restriction of intramolecular rotation was observed.•The thermal stability, electrochemical properties were reported.•Quantum chemistry computation was conducted using the DFT.
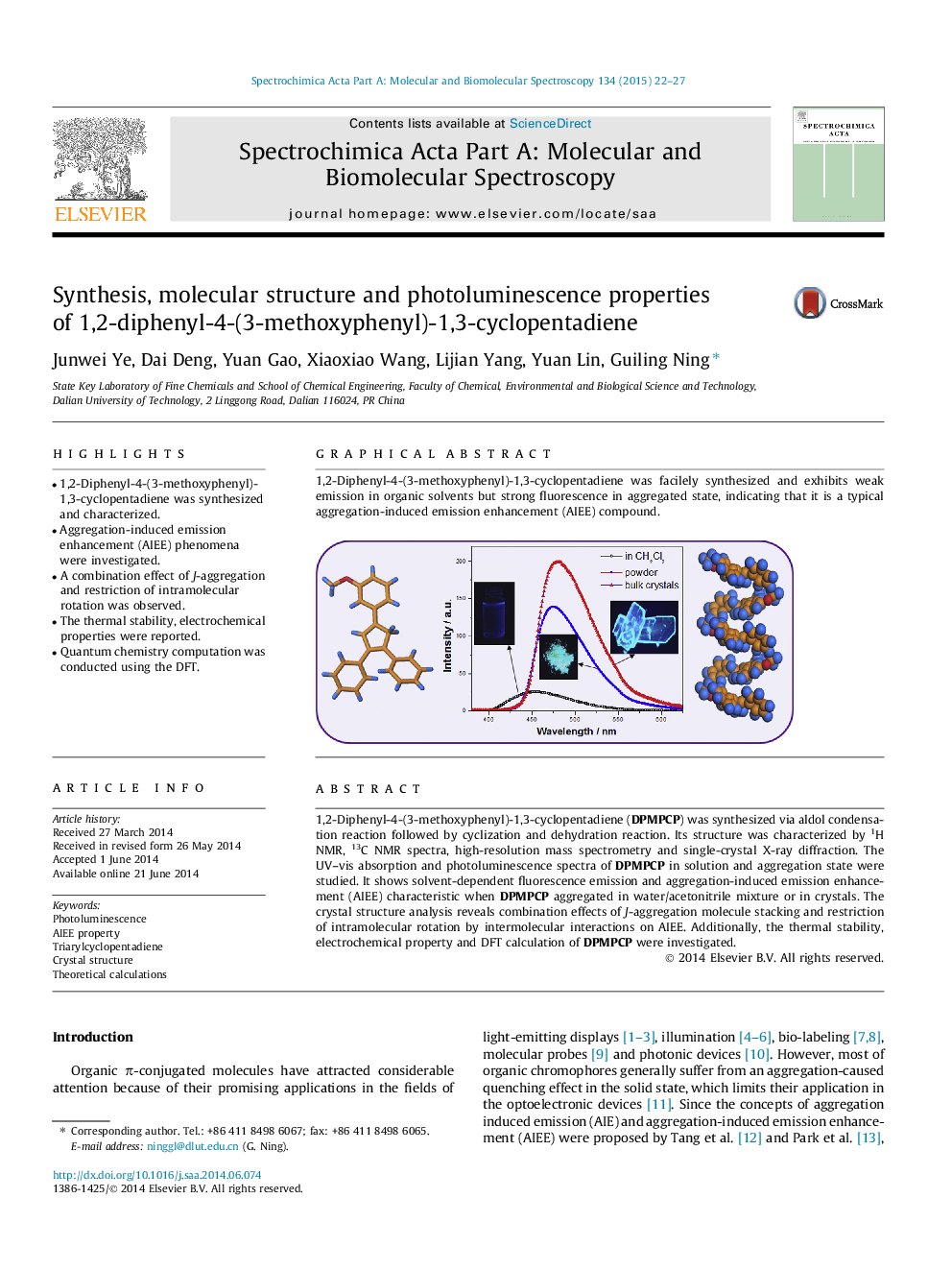
1,2-Diphenyl-4-(3-methoxyphenyl)-1,3-cyclopentadiene (DPMPCP) was synthesized via aldol condensation reaction followed by cyclization and dehydration reaction. Its structure was characterized by 1H NMR, 13C NMR spectra, high-resolution mass spectrometry and single-crystal X-ray diffraction. The UV–vis absorption and photoluminescence spectra of DPMPCP in solution and aggregation state were studied. It shows solvent-dependent fluorescence emission and aggregation-induced emission enhancement (AIEE) characteristic when DPMPCP aggregated in water/acetonitrile mixture or in crystals. The crystal structure analysis reveals combination effects of J-aggregation molecule stacking and restriction of intramolecular rotation by intermolecular interactions on AIEE. Additionally, the thermal stability, electrochemical property and DFT calculation of DPMPCP were investigated.
Graphical abstract1,2-Diphenyl-4-(3-methoxyphenyl)-1,3-cyclopentadiene was facilely synthesized and exhibits weak emission in organic solvents but strong fluorescence in aggregated state, indicating that it is a typical aggregation-induced emission enhancement (AIEE) compound.Figure optionsDownload full-size imageDownload as PowerPoint slide