| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229686 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 11 Pages |
•Non-linear optical material.•IR, UV spectroscopy and single crystal XRD.•Ab-initio calculations.•Squaric acid and CAHB interactions.•Supramolecular architectures.
The experimental and theoretical investigation results of a novel organic squarate salt of 4-Morpholinium bis(hydrogen squarate) (1), C6H14ON+·C8H3O8-, were reported in this study. The crystal structure of the title compound was found to crystallize in the triclinic P − 1 space group. In the crystals of 1 the morpholine ring adopts the chair conformation with the ethyl group in the equatorial and hydrogen atoms in axial positions. The hydrogen squarate anions are linked into a homoconjugated anion, [(HSQ)2H], by a short symmetric, nonlinear O8⋯H2⋯O2 hydrogen bond of 2.444 (2) Å. The structural and vibrational properties of the compound were also studied by computational methods of ab-initio performed on the compound at DFT/B3LYP/6-31++G(d,p) (2) and HF/6-31++G(d,p) (3) level of theory. The obtained calculation results on the basis of two models for both the optimized molecular structure and vibrational properties for the 1 obtained are presented and compared with the X-ray analysis result. On the other hand the molecular electrostatic potential (MEP), electronic absorption spectra, frontier molecular orbitals (FMOs), conformational flexibility and non-linear optical properties (NLO) of the title compound were also studied at the 2 level and the results are reported. In order to evaluate the suitability for NLO applications thermal analysis (TG, DTA and DTG) data of 1 were also obtained.
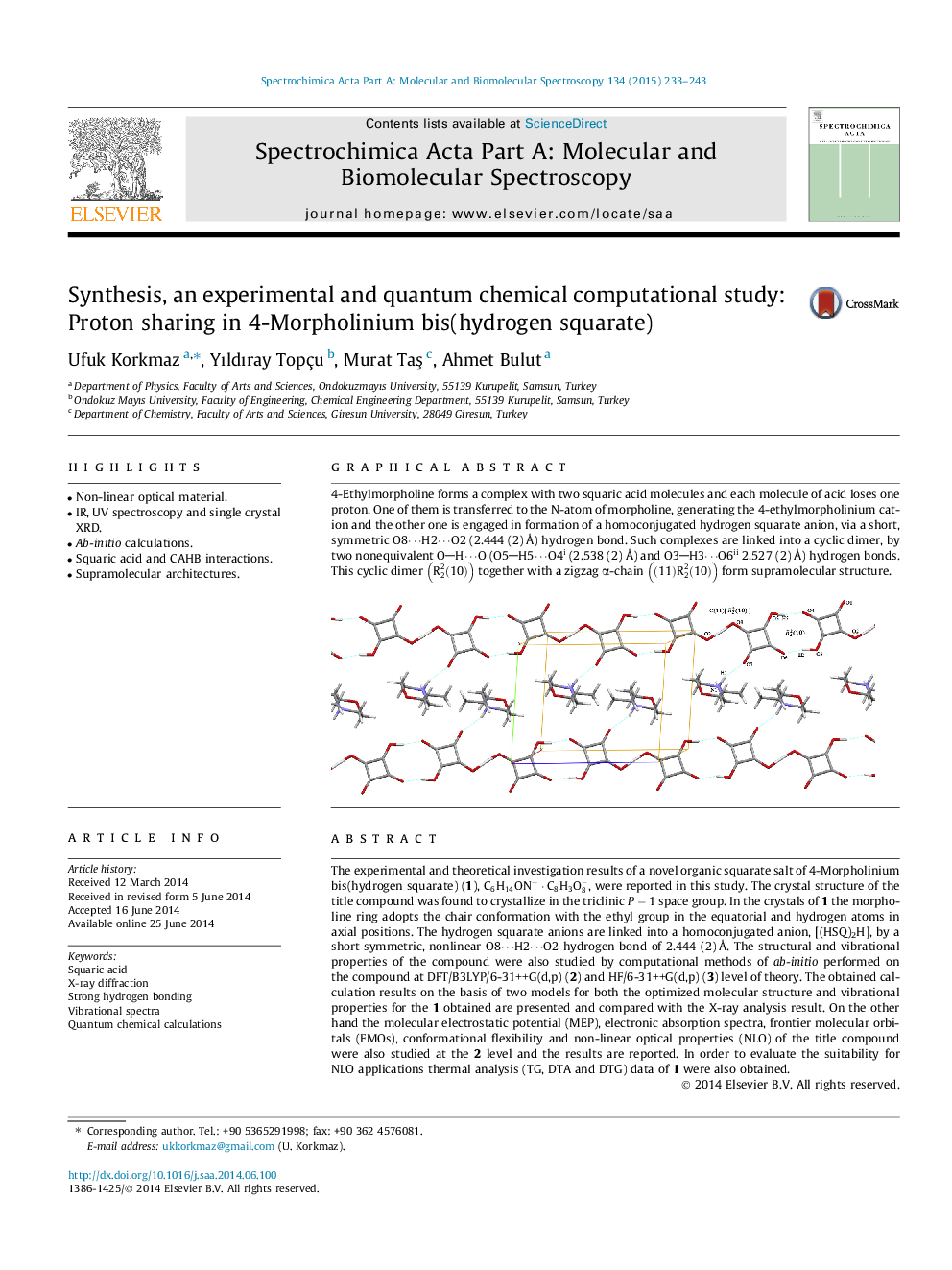
Graphical abstract4-Ethylmorpholine forms a complex with two squaric acid molecules and each molecule of acid loses one proton. One of them is transferred to the N-atom of morpholine, generating the 4-ethylmorpholinium cation and the other one is engaged in formation of a homoconjugated hydrogen squarate anion, via a short, symmetric O8⋯H2⋯O2 (2.444 (2) Å) hydrogen bond. Such complexes are linked into a cyclic dimer, by two nonequivalent OH⋯O (O5H5⋯O4i (2.538 (2) Å) and O3H3⋯O6ii 2.527 (2) Å) hydrogen bonds. This cyclic dimer R22(10) together with a zigzag α-chain (11)R22(10) form supramolecular structure.Figure optionsDownload full-size imageDownload as PowerPoint slide