| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 1229692 | Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy | 2015 | 5 Pages |
•A red phosphor CaAl4O7:Eu3+ has been synthesized by Pechini method.•The photoluminescence properties were found to be influenced by the calcination temperature of the phosphors.•The excitation wavelength and the narrow emission properties suggest their potential application in near-UV white LEDS.
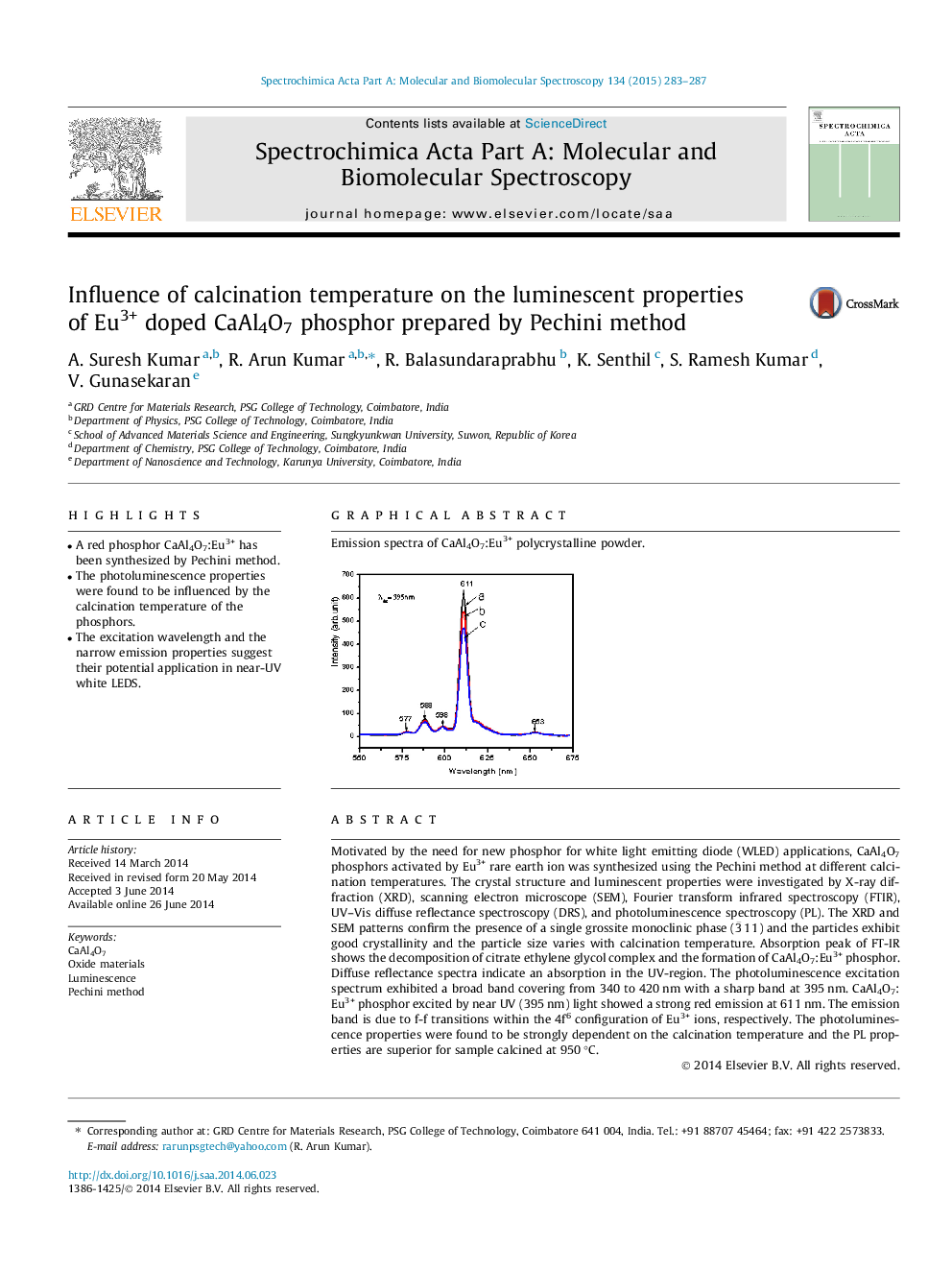
Motivated by the need for new phosphor for white light emitting diode (WLED) applications, CaAl4O7 phosphors activated by Eu3+ rare earth ion was synthesized using the Pechini method at different calcination temperatures. The crystal structure and luminescent properties were investigated by X-ray diffraction (XRD), scanning electron microscope (SEM), Fourier transform infrared spectroscopy (FTIR), UV–Vis diffuse reflectance spectroscopy (DRS), and photoluminescence spectroscopy (PL). The XRD and SEM patterns confirm the presence of a single grossite monoclinic phase (3¯11) and the particles exhibit good crystallinity and the particle size varies with calcination temperature. Absorption peak of FT-IR shows the decomposition of citrate ethylene glycol complex and the formation of CaAl4O7:Eu3+ phosphor. Diffuse reflectance spectra indicate an absorption in the UV-region. The photoluminescence excitation spectrum exhibited a broad band covering from 340 to 420 nm with a sharp band at 395 nm. CaAl4O7:Eu3+ phosphor excited by near UV (395 nm) light showed a strong red emission at 611 nm. The emission band is due to f-f transitions within the 4f6 configuration of Eu3+ ions, respectively. The photoluminescence properties were found to be strongly dependent on the calcination temperature and the PL properties are superior for sample calcined at 950 °C.
Graphical abstractEmission spectra of CaAl4O7:Eu3+ polycrystalline powder.Figure optionsDownload full-size imageDownload as PowerPoint slide